The Doctoral Programme in Drug Research (DPDR) Curriculum
Programme profile
The aim of DPDR is t o educate experts having wide view of the whole life cycle of medicines and toxicology.
o educate experts having wide view of the whole life cycle of medicines and toxicology.
The main doctoral education is research, which is performed in the research groups under guidance of supervisor(s). During research work, also soft skills are learned. These skills can also be studied in the doctoral school’s training events.
DPDR is a multidisciplinary doctoral programme and its strong fields include drug design and synthesis, medicinal chemistry, bioactivity screening, pharmaceutical microbiology, drug formulation, industrial manufacturing, pharmacokinetics, pharmacodynamics, analytics, drug interactions, pharmacogenetics, pharmacology and clinical pharmacology, clinical drug research, pharmacoepidemiology, clinical pharmacy, pharmacoeconomics, toxicology and clinical toxicology and veterinary drug research.
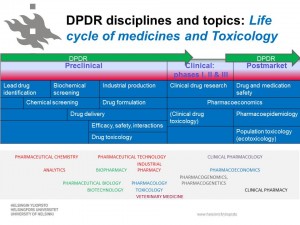 Research topics and disciplines in DPDR.
Research topics and disciplines in DPDR.
Doctoral education in DPDR includes national and international courses, congresses and work visits out-side own working place. DPDR has wide network of experts representing several fields in drug research and toxicology. DPDR is one core unit in the national FinPharmaNet network, which covers seven uni-versities, representatives from national authorities and drug industry. FinPharmaNet organises various educational national events. In addition, cooperation with scientific societies and doctoral candidates’ own activities (Student council) and peer support helps in networking. Research expertise, other skills and career planning are followed in annual thesis committee meetings where outside scientific experts support the progress of doctoral candidates.
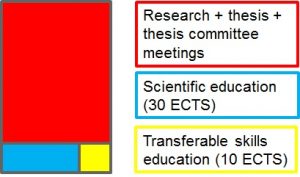
Doctoral education consists of
1) Research (>75 %), including:
– typically 3-5 publications in international, peer reviewed journals (or a monograph)
– (inter)national mobility (see: Grants), networking (e.g. scientific societies)
– Doctoral thesis (a summary of the research including publications), which has to be defended in public.
Doctoral school in health sciences thesis series, instructions for publication: ‘Dissertationes Scholae Doctoralis Ad Sanitatem Investigandam Universitatis Helsinkiensis’.
2) Theoretical education in transferable skills and scientific topics
– Selected transferable skills courses: philosophy of science, statistics, pedagogy, business, IPR, writing and presentation courses (provided primarily by Doctoral School in Health Sciences, DSHealth). Minimum of 10 ECTS of transferable skills education is required for those candidates who start after 1.8.2017.
– Scientific courses and events (organized by DPDR and collaborators) in topics relevant to the Doctoral candidate’s own research but also other topics represented in DPDR in drug research and toxicology. Minimum of 30 ECTS of scientific education, including research ethics (min 1 ECTS), is required for those candidates who start after 1.8.2017.
3) Meetings with thesis committee/follow-up group annually. DPDR does not require compilation of thesis committee (=follow-up group) and annual meetings of those candidates who have registered to pursue doctoral degree before 1.1.2014. For others it is obligatory.
Briefly, updates of research plan, study plan (and TUHAT database) and career prospects are presented to thesis committee/follow-up group to evaluate the progress of doctoral education. DPDR Coordinator is informed with a form. See DPDR guideline (.pdf, updated 25.8.2017) for details.
Note update to DPDR guideline: at least one of the thesis committee members shall not be in the same faculty as the corresponding research group.
4) Potentially Doctoral candidates have other duties, such as teaching and administration.
Doctoral education timeline:
| Year: |
1 |
2 |
3 |
4 |
| – Research – courses – mobility – thesis committee – annual report (TUHAT) |
– Research – courses – mobility – thesis committee – annual report (TUHAT) |
– Research – courses – mobility – thesis committee – annual report (TUHAT) |
– Research – courses – mobility – thesis committee – annual report (TUHAT) – thesis writing – dissertation – PhD degree |
Description of DPDR and curriculum as pdf, 1_Opetussuunnitelma_DPDR.
See also corresponding Faculty information:
and overall doctoral education in DSHealth handbook and University instructions.
Transition to new degree requirements
Transition from the previous degree requirements to the requirements starting on 1.8.2017: General transition guidelines 2017-2020 DPDR.
Suitable courses for doctoral degree in drug research and toxicological research
1) University of Helsinki
A collection of Scientific courses in DPDR is listed below (new update will be published in 2017).
Transferable skills are provided by Doctoral School in Health Sciences (DSHealth). Also, it’s Doctoral programmes organise scientific and transferable skills training.
Courses in English by the Faculty of Pharmacy are listed in the Faculty page. Also additional Faculty of Medicine courses can be found via faculty web site.
Description and curriculum of DPDR (as .pdf)
2) National courses
National drug research training network FinPharmaNet (earlier FinPharma Doctoral Program, FPDP) organises Annual meeting which is highly recommended for all doctoral candidates and supervisors. FinPharmaNet announces scientific training courses in drug research and toxicology organised by Finnish Universities (especially drug research doctoral programmes in the Universities of Eastern Finland and Turku) and by other organisers.
See also national course list in FinBioNet page (→ FinBioNet course calendar).
3) International courses
NorDoc – Nordic Doctoral Training in Health Sciences network provides doctoral training courses offered by the NorDoc partner organisations:
-
Faculty of Medicine and Dentistry, University of Bergen (Norway)
-
The Graduate School of Health and Medical Sciences, University of Copenhagen (Denmark)
-
Doctoral School in Health Sciences, University of Helsinki (Finland)
-
Health and Biosciences Doctoral Programme, University of Oulu (Finland)
-
Graduate School of Health Sciences, University of Southern Denmark (NOT AVAILABLE YET)
-
Faculty of Medicine Postgraduate Education Unit (PGE), University of Turku
For the Doctoral candidates in the Faculty of Pharmacy, ULLA network courses with Faculty support for travel are available.
See also News in DPDR home page.
Scientific courses in DPDR
Most, but not all, courses are listed in WebOodi.
Topics:
General education
Lead drug identification, lead optimisation
Chemical screening
Biochemical screening
Drug delivery
Biotechnology
Drug formulation
Industrial production
Efficacy, safety, interactions
Drug toxicology
Clinical drug research
Clinical drug toxicology
Pharmacoeconomics
Drug and medication safety
Pharmacoepidemiology
(Population and environmental toxicology)
Veterinary medicine
Updated 1.2.2016. Course codes and contents may be changed in Autumn 2017.
General education
| OODI code | Course name | Practise | ECTS | Duration | Organiser | Dates | times/ 4 years |
| 590381 | Preliminary exam (in pharmaceutical sciences) | 6-8 | Faculty of Pharmacy | 2016 x 4 | 8 | ||
| Defense of the research plan (before 2014 registered Doctoral candidates) | 3 | Doctoral candidate | on demand | ||||
| 590382 | Presentation of the dissertation research project I (in pharmaceutical sciences) | 2 | 2016 | ||||
| 590383 | Presentation of the dissertation research project II (in pharmaceutical sciences) | 2 | 2016 | ||||
| FPDP/FinPharmaNet Annual Meeting , Turku (Helsinki/Kuopio/Tampere/Oulu) | DRDP/UTU | 29.-30.8.2016 | Annual | ||||
| Viikki Journal clubs and seminar series | http://www.biocenter.helsinki.fi/events.html | Annual | |||||
| Biomedicum events | http://www.biomedicum.fi/index.php?page=116&lang=2 | Annual |
Lead drug identification, lead optimisation
| OODI code | Course name | Practise | ECTS | Duration | Organiser | Dates | |
| Practical course on G protein-coupled receptors (Oulu) | lectures, practicals | 5 days | Henri Xhaard | – | |||
| Graduate student workshop: Computational methods in protein science | 1 | 2 days | Henri Xhaard | – | |||
| G protein coupled receptors (GPCRs201X), Helsinki | 4 days | Henri Xhaard | – | ||||
| FinMedChem 2017, Helsinki | Lectures + exercises | 2 | 2 days | Jari Yli-Kauhaluoma, Erik Wallen | – | Every other year | |
| 590280 | Modeling protein-ligand complexes (MPLC) | Lectures + exercises | 3 | Henri Xhaard | – | Every other year | |
| 590334 | Chemoinformatics | Henri Xhaard | – | Every other year | |||
| 590173 | Organic chemistry in molecular biosciences and pharmacy III | Lectures | 3 | Jari Yli-Kauhaluoma | 2016 | ||
| 590321 | Structure elucidation of organic compounds | Lectures | 2 | Hannu Elo | 2016 | ||
| 590219 | Organic chemistry of enzyme-catalyzed reactions | 3 | Jari Yli-Kauhaluoma | – | |||
| 55727 | Basic Course in Radiochemistry | Lectures | 4 | Risto Harjula | Annual | ||
| 55722 | Radiation Protection | Lectures + excercises | 2 | Risto Koivula | Annual | ||
| 55742 | Radiopharmaceutical chemistry | Lectures + excercises | 3 | Anu Airaksinen | – | Every other year | |
| 55782 | Tracer techniques | Lectures + excercises | 3 | Anu Airaksinen | 2016 | Every other year | |
| 590337 | Applied mathematical tools for pharmaceutical research | 2 | Alex Bunker | 2016 | Annual | ||
| 590287 | Fundamentals of Molecular Modeling and Molecular Dynamic Simulations in Pharmaceutical Research | 5 | Alex Bunker | 2016 | Annual | ||
| 590384 | Anslyn, E. V. & Dougherty, D. A., Modern Physical Organic Chemistry, University Science Books, 2005, 1100 pp. | Book exam | 11 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Bugg, T. D. H., Introduction to Enzyme and Coenzyme Chemistry, 3rd. Edition, Wiley-Blackwell, 2012, 290 pp. | Book exam | 3 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Carey, F. A. & Sundberg, R. J., Advanced Organic Chemistry, Part A: Structure and Mechanisms, 5. Edition, Springer, New York, 2007, 1199 pp. | Book exam | 12 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Carey, F. A. & Sundberg, R. J., Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5. Edition, Springer, New York, 2007, 1321 pp. | Book exam | 13 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Corey, E. J. & Czakó, B. & Kürti, L, Molecules and Medicine, Wiley, Hoboken, 2007, 254 pp. | Book exam | 3 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Dewick, P. M.: Medicinal Natural Products – A Biosynthetic Approach, Wiley, 2009, 3. Edition, 539 pp. | Book exam | 5 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Lemke, T. L., Williams, D. A., Roche, V. F. & Zito, S. W., Foye’s Principles of Medicinal Chemistry, 7th Edition, Lippincott Williams & Wilkins, Philadelphia, 2013, 1520 pp | Book exam | 5-15, by agreement | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | McMurry, J. & Begley, T., The Organic Chemistry of Biological Pathways, Roberts & Company Publishers, Greenwood Village, 2005, 490 pp. | Book exam | 5 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Nogrady, T. & Weaver, D. F., Medicinal Chemistry, A Molecular and Biochemical Approach, Oxford University Press, Oxford, 2005, 649 pp. | Book exam | 6 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Patrick, G. L., An Introduction to Medicinal Chemistry, Oxford University Press, 5. Edition, Oxford University Press, Oxford, 2013, 789 pp. | Book exam | 8 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Silverman, R. B. & Holladay, M. W., The Organic Chemistry of Drug Design and Drug Action, 3. painos, Elsevier Academic Press, San Diego, 2014, 517 pp. | Book exam | 5 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Silverman, R. B., The Organic Chemistry of Enzyme-Catalyzed Reactions, Academic Press, San Diego, 2000, 717 pp. | Book exam | 7 | Jari Yli-Kauhaluoma | 2016 | ||
| 590384 | Wermuth, C. G., The Practice of Medicinal Chemistry, Academic Press, 3. Edition, San Diego, 2008, 942 pp. | Book exam | 8 | Jari Yli-Kauhaluoma | 2016 | ||
| Zweifel, G. S. & Nantz, M. H., Modern Organic Synthesis, An Introduction, Freeman, New York, 2007, 477 pp. | Book exam | 5 | Jari Yli-Kauhaluoma | 2016 |
Chemical screening
| 590134 | Bioanalytics | Lectures | 5 | Risto Kostiainen | 2016 | ||
| 590085 | Drug metabolism | Lectures | 3 | Risto Kostiainen | 2016 | ||
| 590179 | Mass spectrometry | Lectures | 3 | Tapio Kotiaho | 2016 | Every other year | |
| 55272 | Modern carbohydrate analysis | Lectures | 2 | Helena Soini | |||
| 590243 | Optimization of Chromatographic Methods | Lectures | 3 | Heikki Vuorela | 2016 |
See Department of Chemistry (Faculty of Science) courses in OODI
Biochemical screening
| 590283 | Essentials of Molecular Biology in Pharmacy | Lectures | 3 | Moshe Finel | 2017 | Every other year | |
| 590323 | Natural Substances as Medicines | Luentokurssi | 5 | Yvonne Holm | 2016 | ||
| 590284 | Cells – Biomaterials interactions, Pharmaceutical and medical applications in biopharmacy | Lectures | 3 | 1 month | Yan-ru Lou | 2016 | Annual |
| 590320 | Isolation and Analysis of Natural Compounds | Luentokurssi | 5 | Heikki Vuorela | |||
| 590385 | Microbial lifestyles and drug discovery of antimicrobials | Lectures, seminars and lab/groupworks | 5 | Adyary Fallarero | 2017 |
Biotechnology
| P4 Medicine | course/symposium | Repasky/FIMM | 2017 | Every three years | ||
| Bioinformatics in Pharmacogenomics | lectures and hands-on training | Tero Aittokallio/FIMM | – | |||
| Computational Systems Medicine with focus on Network Pharmacology | Tero Aittokallio/FIMM | – | ||||
Drug delivery
| 590237 | Advanced Biopharmaceutics, Lectures | Lectures | 3 | Marjo Yliperttula | 2016 | Annual | |
| 590296 | Introduction to nanosciences – Johdatus nanotieteeseen | Lectures | 4 | Tapani Viitala | Spring 2016 | Annual | |
| 590329 | Advanced course in pharmacokinetics I | Lectures | 3,5 | Arto Urtti | 2016 | ||
| 590297 | Nanoforum | symposium | 2 | Vincenzo Cerullo | 2017 | ||
| Membrec | symposium | 0.5+0.5 | Pia Siljander/Fac. Biol. Env. Sci | 8.9.2016 |
Drug formulation
| Microfluidics and nanotechnology for pharmaceutical applications (MiNaPharmA) | Lectures(+lab) | 3-5 | Helder Santos, Tiina Sikanen |
2017, 2019
|
|||
| 59072 | Controlled Drug Delivery | Lectures | 3 | Jouni Hirvonen | 2016 | ||
| 590176 | Solid state analysis of pharmaceutics | Lectures | 3 | Clare Strachan | |||
| 59067 | Product Development and Experimental Design | Lectures | 3 | Jouko Yliruusi | |||
| 590234 | Physical Pharmacy | Lectures | 5 | Marjo Yliperttula, Jouko Yliruusi | |||
| 59068 | Excipients in Pharmaceutical Technology | Lectures | 3 | Leena Peltonen | |||
Industrial production
| 590211 | Expert Leadership | Lectures | 4 | Anne Juppo | – | |
| 590278 | Biopharmaceutics in Pharmaceutical Product Development – Biofarmasia farmaseuttisessa tuotekehityksessä | Book exam | 3 | 4 days | Mia Siven | Annual |
| 590212 | Business economy of pharmaceutical industry | Book exam | 3 | Anne Juppo | Annual | |
| 590332 | Special characteristics of use and development of veterinary medicines | Book exam | 4 | Mia Siven | Annual | |
| 590153 | Formulation I | Book exam | 5 | Anne Juppo | Annual | |
| 590154 | Formulation II | Book exam | 4 | Anne Juppo | Annual | |
| 590155 | Formulation III | 6 | Anne Juppo | 2016 | ||
| 590160 | Quality management and quality standards | Book exam | 6 | Anne Juppo | Annual | |
| 590367 | Pharmaceutical marketing | Book exam | 3 | Anne Juppo | Annual | |
| 590210 | Good manufacturing and distribution practice | 7 | Anne Juppo | 2017? | ||
| 590162 | Pharmaceutical industry and wholesale operations | 4 | Mia Siven | 2016 | ||
Efficacy, safety, interactions
| Stereotaxis in neuropharmacological research | lectures, exam, practical | 0,5-3 | 1-2 days | Petteri Piepponen | – | |
| Advances in Heart Regeneration and Repair | 2 days | Heikki Ruskoaho | – | |||
| 59084 | Neuropharmacology | Lectures | 3 | Raimo Tuominen | 2016 | |
| Bioinformatics in Pharmacogenomics | lectures and hands-on training | FIMM | 2016 | |||
| Computational Systems Medicine with focus on Network Pharmacology | FIMM | 2017 | ||||
| Gene manipulation – Advanced tools for drug research | 1 | 2 days | Outi Salminen | ? | ||
| BEHAVIOURAL PHENOTYPING OF RODENT DISEASE MODELS – POTENTIAL AND PITFALLS | Lectures, demonstrations | 2-3 | 6 days | Voikar/UH, Tanila, Jolkkonen/UEF, Vasar/U Tartu | 28.8.–2.9.2016 | |
| Neuropharmacology of drug addictions | Saara Nuutinen | – |
Drug toxicology
Clinical drug research
| Pharmcokinetics, hands on (Introduction to Phoenix Winnonlin) | Aleksi Tornio | ? | Every 2-3 years | ||||
| Kliininen lääketutkimus | Lectures | Janne Backman | – | Every 2-3 years | |||
| Lääkkeiden haittavaikutukset – mitalin toinen puoli / Drug interactions | Luennot | Janne Backman | Every 2-3 years | ||||
| Pharmacogenetics | Lectures | Mikko Niemi | ? |
Clinical drug toxicology
Pharmacoeconomics
| 590300 | Critical appraisal of evidence | Lectures, exercises, assignments | 5 | 1 day | Marja Blom | Annual |
| 590311 | Vaikuttavuuden mittaaminen farmakoekonomisessa tutkimuksessa – Measuring effectiveness in health care research | Lectures, exercises, assignments | 4 | 2 days | Marja Blom | 2017 |
| 590372 | Research methods in pharmacoeconomics | Lectures, exercises, assignments | 5 | Marja Blom | Annual | |
| 590376 | Literature in pharmacoeconomics | Literature will be agreed case by case basis | 1-6 | Marja Blom | Annual |
Drug and medication safety
| 590301 | Clinical pharmacy and medication management in hospital | Lectures | 6 | Marja Airaksinen | |||
| 590183 | Drug Information and Drug Information Services | Lectures | 4 | 0 | Marja Airaksinen, Niina Mononen, Marika Pohjanoksa-Mäntylä | 2016 | Every other year |
| 590250 | Medication review and clinical pharmacy | Lectures | 4 | Marja Airaksinen, Niina Mononen, Marika Pohjanoksa-Mäntylä | 2016 | ||
| 590386 | High risk medicines and medicine induced disorders | Lectures | 4 | Raisa Laaksonen | |||
| Medication safety research – Overview on Methods and Current Topics | lectures + diary | 1 | 1 day | Marja Airaksinen | |||
| Statistics for Health Services Research: Multivariable Methods | assignments | 3 | 2 days | Marja Airaksinen | |||
| Outcomes Assessment, Rational Drug Therapy and Pharmacy (Lääkehoidon tuloksellisuuden arviointi ja rationaalinen lääkehoito) | 2 or 4 | 2 days | Marja Airaksinen |
Pharmacoepidemiology
(Population and environmental toxicology)
Not available at the moment
Veterinary medicine
| Doctoral programme in clinical veterinary medicine courses | DPCVM web site |
Welcome to candidate (.pdf) to new members of DPDR (updated 10.8.2016).
The University of Helsinki PhD Students is an association for all doctoral candidates at the University of Helsinki. The goal is to advocate the interests of doctoral students and to function as a liaison between doctoral students and the University of Helsinki.
Note: Some of the doctoral education (transferable skills or scientific) courses are very popular and there is a long queue for the courses. In case registered participant does not cancel his/her but does not show on a course someone misses the opportunity to participate.
Therefore, if doctoral candidate does not show on two courses organized despite registration (and without cancellation) by the DSHealth/Doctoral programme within 12 months, he/she is not entitled to DSHealth/Doctoral programme travel grants in the next application round.
Revised edition of European Code of Conduct for Research Integrity 2017 (as pdf)