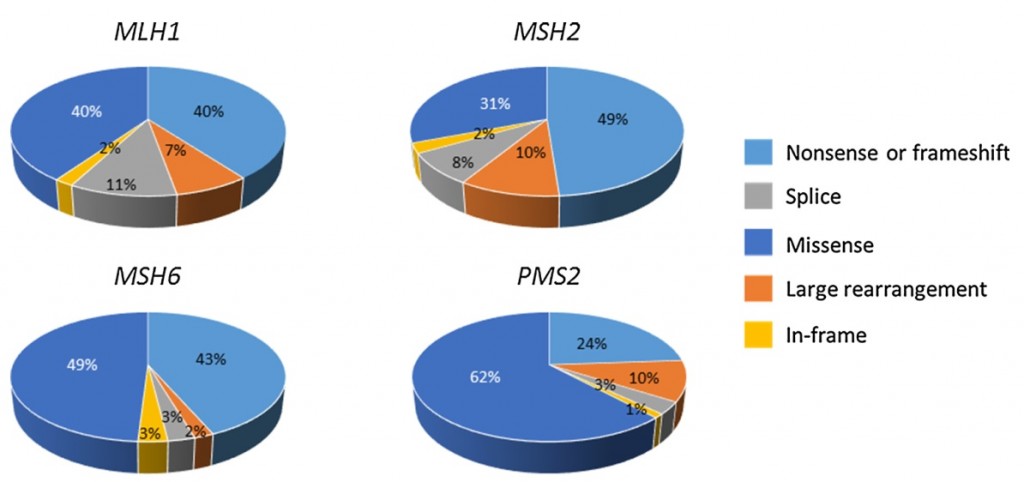
Itkonen HM, Kantelinen J, Vaara M, Parkkinen S, Schlott B, Grosse F, Nyström M, Syväoja JE, Pospiech H.
The high fidelity of genome duplication is ensured by cooperation of the proofreading and mismatch repair (MMR) activities of DNA polymerases. Here, we show that human mismatch recognizing proteins MutS homologue 2 (MSH2) and MSH6 co-purify and interact with replicative Pol α, the replicative primase which replicates DNA with poor fidelity. We show that MSH2 associates with known human replication origins with different dynamics than Pol α. Furthermore, we explored the potential functional role of Pol α in the mismatch repair reaction by using an in vitro mismatch repair assay and observed that Pol α promotes mismatch repair. Taken together, we show that human Pol α interacts with MSH2-MSH6 complex and propose that this interaction occurs during the mismatch repair reaction.
FEBS Letters, 2016