by Mingzhen Zhang
Arctic temperatures are warming more than twice as fast as the global average [1, 2], causing permafrost thaw, loss of ice and snow cover on land and over the sea. Moreover, feedbacks from these changes aggravate global warming; the phenomenon is termed Arctic amplification. These changes attract increasing public attention, which is centered at the shrinking ice-cover over the ocean, the melting ice sheets and glaciers and thawing permafrost. Increasing research interest, however, is devoted to studying arctic lakes, which make up 25% of the world’s laketotal and cover up to 50% of the Arctic terrestrial landscape [3, 4]. For the public, the nature and importance of arctic lakes has remained vague. In this post I will try to elucidate some facts based on my own experiences.
Arctic lakes make up 25% of the world’s lakes and cover up to 50% of the arctic terrestrial landscape
- The uniqueness of arctic lakes
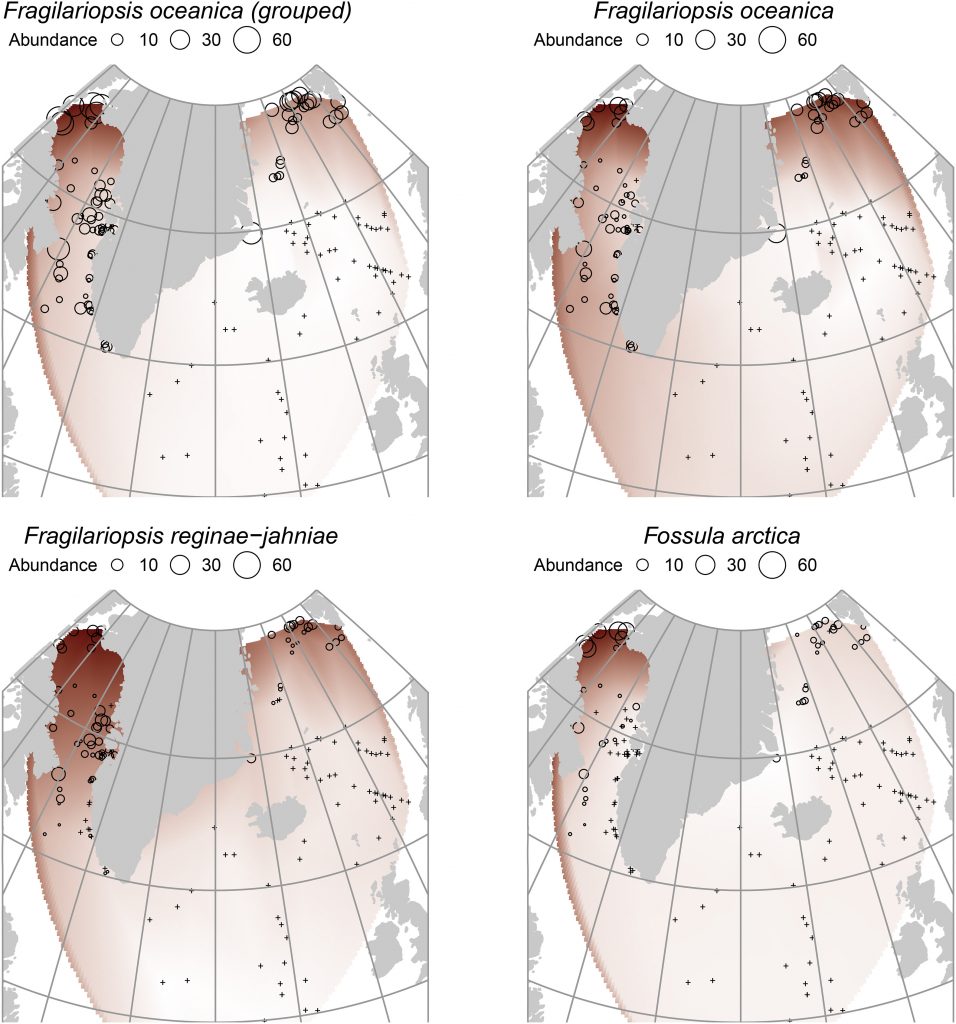
Before I planned to move to Finland for my doctoral research, “arctic lakes” were just two simple words for me. I only knew that these lakes were within the Arctic Circle. (Now I also know that anything northwards the Arctic Circle is only one definition of “arctic”.) Although I had some research experience on eutrophic Chinese lakes from my Master’s program, I felt unfamiliar with arctic lakes, which are significantly different from urban lakes I investigated previously. First, in terms of physical and chemical characteristics, urban lakes usually keep relatively stable states over the year, occasionally fluctuating with extreme weather events. In contrast, due to the pronounced climate, changing snow and ice cover, and differences in size, elevation, morphometry and ways of formation, arctic lakes experience a range of seasonal fluctuations in temperature, light availability and water quality [3]. Second, considering biological communities and production, urban lakes have higher species diversity involving all kinds of bacteria, phytoplankton, zooplankton, benthic species, fish and macrophytes, together with overall high primary productivity (dense algal blooms), compared to arctic lakes which are generally characterized by few species and low productivity [5]. The above-mentioned general characteristics of arctic lakes usually refer to lakes with clear water. However, arctic lake scenery is highly variable (Fig. 1). There are thermokarst lakes formed due to local permafrost degradation, and meltwater lakes, receiving glacial runoff with large amounts of suspended silt, and their water properties are very different from the clear-water lakes. Majority of the dozens of different ways for lake formation are presented in the Arctic, giving rise to the unique and diverse properties of arctic lakes, many of which remain unstudied. Arctic freshwater ecosystems provide services for arctic people and fauna. They are also important for global biodiversity, energy balance and carbon budget, all of which are rapidly changing due to climate warming.
Figure 1. A variety of lakes in northernmost Finland. (a) Lake Saanajärvi at the altitude of 679 meters. (b) A lake surrounded by mountain birch forest at the altitude of 553 m. (c) Lake Kilpisjärvi at the altitude of 473 m. (d) An arctic lake without the official name at the altitude of 1009 m. (e) Lake Jeahkatslampi at the altitude of 930 m. (f) Lake Bahtasgohpejavri at the altitude of 776 m. Photos courtesy of Jan Weckström (a, c, d, e) and Maija Heikkilä (b, f), Environmental Change Research Unit.
2. The impact of climate change on arctic lakes
Climate change has a multitude of direct and indirect effects on arctic lake ecosystems. Changes in air temperatures, wind and precipitation patterns affect the structure, function and biodiversity of lakes, but so do changes in freshwater, nutrient and sediment inputs from lake catchment areas. First, continuously increasing air temperatures induce increasing lake water temperatures, which can change the thermal regimes of lakes, such as variation in mixing and stratification patterns. Consequently, many biogeochemical and biological processes and species composition of the lakes are affected [6]. Second, glacier retreat and permafrost thaw lead to more nutrients, organic matter and silt flowing into lakes, potentially modifying nutrient and light conditions in the oligotrophic, clear arctic lakes and resulting in higher primary production in summer and emerging new species [3]. In addition, climate change directly affects lake ice cover. One of the earliest observed impacts of climate warming was based on the loss of freshwater ice [7]. Research evidence demonstrates later lake ice-on and earlier ice-off dates, leading to shorter annual durations of ice cover and thus changes in lake ecology [8, 9]. Although there are many factors (e.g., air temperature, wind speed, snow thickness, solar radiation) that can affect ice formation and melt in shallow and deep lakes, air temperature is the key driver [10]. Thus, lake ice phenology can serve as a useful indicator of late autumn and early spring climate change in a regional scale [11]. Additionally, lake ice plays a vital role in socio-economy such as ice roads, transportation, cultural recreation and tourism [12]. Understanding the freeze-thaw cycles of lake ice and their effects on arctic ecosystems including the human, can promote the safety of the region on many levels.
Research evidence demonstrates later ice-on and earlier ice-off dates of arctic lakes
3. Arctic lakes and past climate reconstruction
Human societies have had an increasing influence on global climate over the past centuries. Understanding natural climate changes and ecosystem responses is quintessential in preparing for future. As I mentioned above, arctic lakes are sensible to climate variability. What is more, their bottom mud or sediments, serve as a chronicle of changes over the past millennia. Lake sediments archive variations in biological and physical conditions and provide a unique temporal record of climatic change [13]. Many indicators, such as microfossils (diatoms, chrysophycean cysts, chironomids, cladocerans), biogeochemical markers (elemental and isotopic geochemistry, plant pigments, plant lipids), mineral magnetic analyses, and various sediment indices (the accumulation rates of organic carbon, nutrients, contaminants, etc.) have been developed to analyze the shifts in lake physical, chemical and ecosystem qualities [13, 14]. Therefore, a comprehensive arctic paleoclimate data network, covering various lake types in various settings, is necessary for reliable assessments of past, present and future climate patterns.
Circumpolar regions might seem isolated from the rest of the planet, but in reality, they play an integral role in the global climate system. Furthermore, these regions have been inhabited by arctic peoples for thousands of years [15], and experienced significant environmental transitions due to climate changes and human impacts. Improved understanding of northern nature and lakes and their responses to environmental changes will contribute to the sustainable development of the Arctic. It is a great honor for me to have an opportunity to participate in this research, and I hope to contribute to elevated knowledge of fascinating arctic lakes.
Mingzhen is a doctoral researcher whose research focuses on arctic lakes in northernmost Finland.
Reference
- Meredith M, et al. 2019. In: IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Cambridge University Press, Cambridge, UK and New York, NY, USA, 2019. 203-320. https://doi.org/10.1017/9781009157964.005.
- Moon TA, et al. (2021). Arctic Report Card 2021. https://www.arctic.noaa.gov/Report-Card/Report-Card-2021
- Jeppesen E, et al., 2020. Ecology of Arctic Lakes and Ponds. In Thomas, DN (ed): Arctic Ecology, pp. 159-180. https://doi.org/10.1002/9781118846582.ch7
- Muster S, et al., 2017. PeRL: a circum-Arctic permafrost region pond and lake database. Earth System Science Data 9: 317–348. https://doi.org/10.5194/essd-9-317-2017
- Christoffersen KS, et al., 2008. Food-web relationships and community structures in high-latitude lakes. In Vincent WF & Laybourn-Parry J (eds): Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems. DOI:10.1093/acprof:oso/9780199213887.003.0015
- Arvola L, et al., 2009. The Impact of the Changing Climate on the Thermal Characteristics of Lakes. In George, G (ed.): The Impact of Climate Change on European Lakes, 85-101. DOI: 10.1007/978-90-481-2945-4
- Walsh SE, et al., 1998. Global patterns of lake ice phenology and climate: Model simulations and observations. Journal of Geophysical Research: Atmospheres 103(D22): 28825-28837. DOI: 10.1029/98jd02275
- Magnuson JJ, et al., 2000. Historical Trends in Lake and River Ice Cover in the Northern Hemisphere. Science 289: 1743-1746. DOI: 10.1126/science.289.5485.1743
- Benson BJ, et al., 2012. Extreme events, trends, and variability in Northern Hemisphere lake-ice phenology (1855–2005). Climatic Change 112: 299–323. DOI: 10.1007/s10584-011-0212-8
- Kirillin G & Leppäranta M, 2021. Lake Ice Formation and Melt. Under-Ice Dynamics. Reference Module in Earth Systems and Environmental Sciences,
Elsevier. https://doi.org/10.1016/B978-0-12-819166-8.00003-7 - Hodgkins GA., et al., 2002. Historical changes in lake ice-out dates as indicators of climate change in New England, 1850-2000. International Journal of Climatology 22: 1819-1827. DOI: 10.1002/joc.857
- Arp CD, 2019. Ice roads through lake-rich Arctic watersheds: Integrating climate uncertainty and freshwater habitat responses into adaptive management Arctic, Antarctic, and Alpine Research 51, 9-23. https://doi.org/10.1080/15230430.2018.1560839
- Korhola A, et al., 2002. A multi-proxy analysis of climate impacts on the recent development of subarctic Lake Saanajärvi in Finnish Lapland. Journal of Paleolimnology 28: 59-77. https://doi.org/10.1023/A:1020371902214
- Lehnherr I, et al., 2018. The world’s largest High Arctic lake responds rapidly to climate warming. Nature Communications 9: 1290. https://doi.org/10.1038/s41467-018-03685-z
- Kotlyakov VM, et al., (eds.) 2017. Human Colonization of the Arctic: The Interaction Between Early Migration and the Paleoenvironment. 530 pp. https://doi.org/10.1016/B978-0-12-813532-7.01001-9