by Ben Redmond Roche
I was lucky enough to be a member of the Heikkilä Research Group during the latter half of my MSc (2018-19) and worked very closely with the sagacious diatom specialist Kaarina Weckström. My MSc project appears of interest to the wider scientific community: since our findings were accepted for publication in Marine Micropaleontology (1) in March 2020 there has been ongoing interest in the work, with 4 citations already (2-5). A year after acceptance, as of March 2021, it is still the most downloaded (within past 90 days) article from the journal! How did my project advance in practice? Why does it matter?
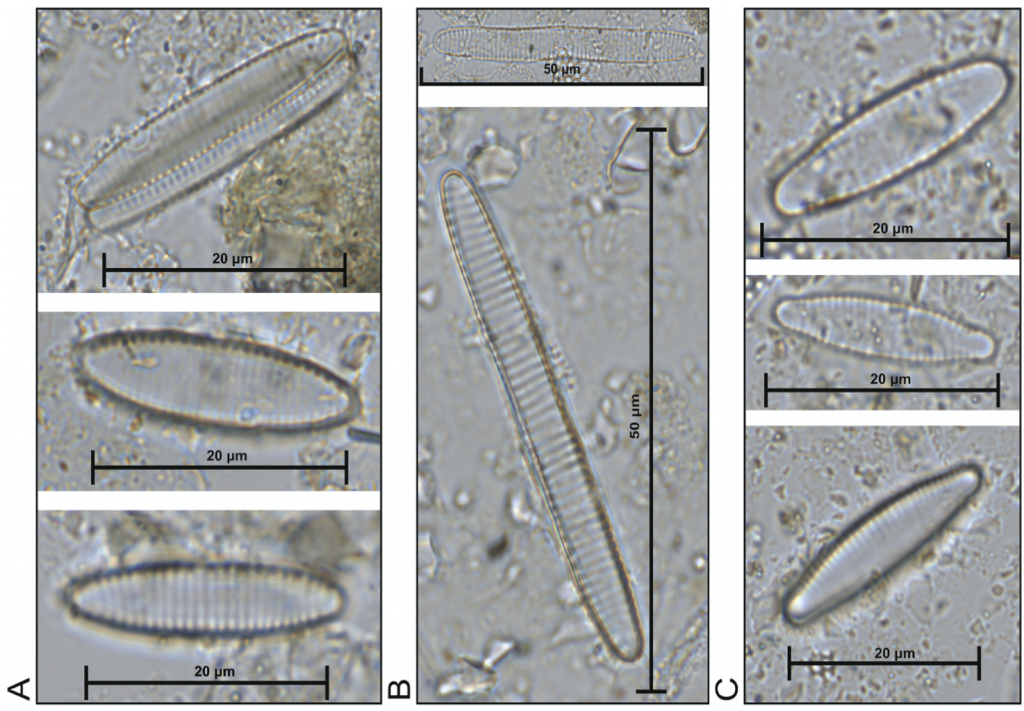
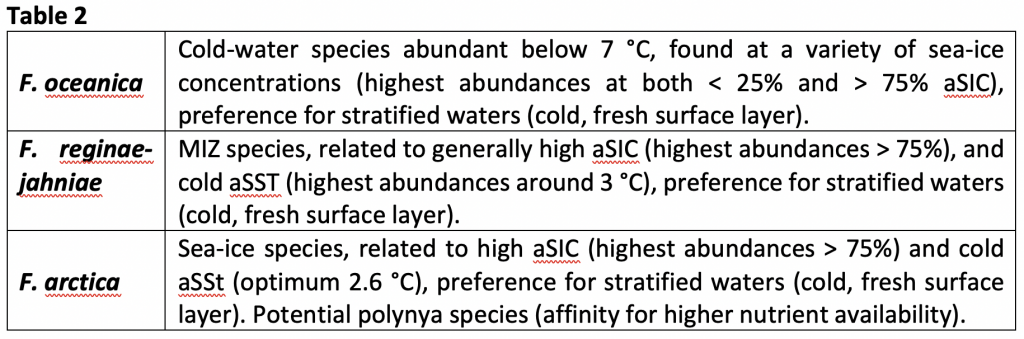
The research comprised re-examining 46 selected microscope slides from the North Atlantic training set (6, 7, 8), the largest and most geographically extensive diatom set in the Northern Hemisphere, in order to explore the spatial distributions of the sea-ice affiliated diatoms Fragliaropsis oceanica, Fragilariopsis reginae-jahniae and Fossula arctica (A, B & C in Fig. 1, respectively) with respect to sea-surface temperatures and sea-ice concentrations. Due to the subtle morphological differences, the previous training set and literature had grouped the three species collectively under one species name, F. oceanica.
Figure 1. 1000x magnification (A) F. oceanica (B) F. reginae-jahniae (3) F. arctica.
In paleoceanography, training sets such as the one I began exploring, are used to assess the modern relationships of microfossil species (in this case diatoms) to environmental conditions, such as sea-surface temperatures or sea-ice concentration. These species-environment relationships can then be used for reconstructing past environmental conditions from the microfossils in sediment sequences covering past millennia.
I began my research project asking: Are the distributions and thus ecologies of the three diatom species different? Does grouping the three species in the training set potentially mask the true signal of past ocean conditions in reconstructions?
Since the three species are morphologically very similar, and I spent several days training on the microscope with Kaarina learning to distinguish them from one another. The differences can be seen in Figure 1 and summarised by the following descriptions:
It took a while to gain competence on the microscope, but eventually I got my eye in and noted the fundamental differences: F. reginae-jahniae is elongated and the valves are more parallel; F. oceanica has more rounded valves; and F. arctica has the idiosyncratic circular ends. I then counted the relative abundance of the species in the 46 slides over several weeks (a good podcast is crucial when counting diatoms!). We then analysed the species-environment relationships, particularly to April sea-ice concentration (aSIC) and August sea surface temperature (aSST). The multivariate statistical analyses encompassed two aspects: a redundancy analysis (RDA), expressed as a biplot where species are plotted against the variables, and environmental response curves of the species created using R. The latter was done by Professor in Quantitative Palaeoecology Steve Juggins from Newcastle University (p.s. If you start working on quantitative reconstructions, I highly recommend the Chapter Steve wrote with John Birks in vol 5 of Tracking Environmental Change Using Lake Sediments.).
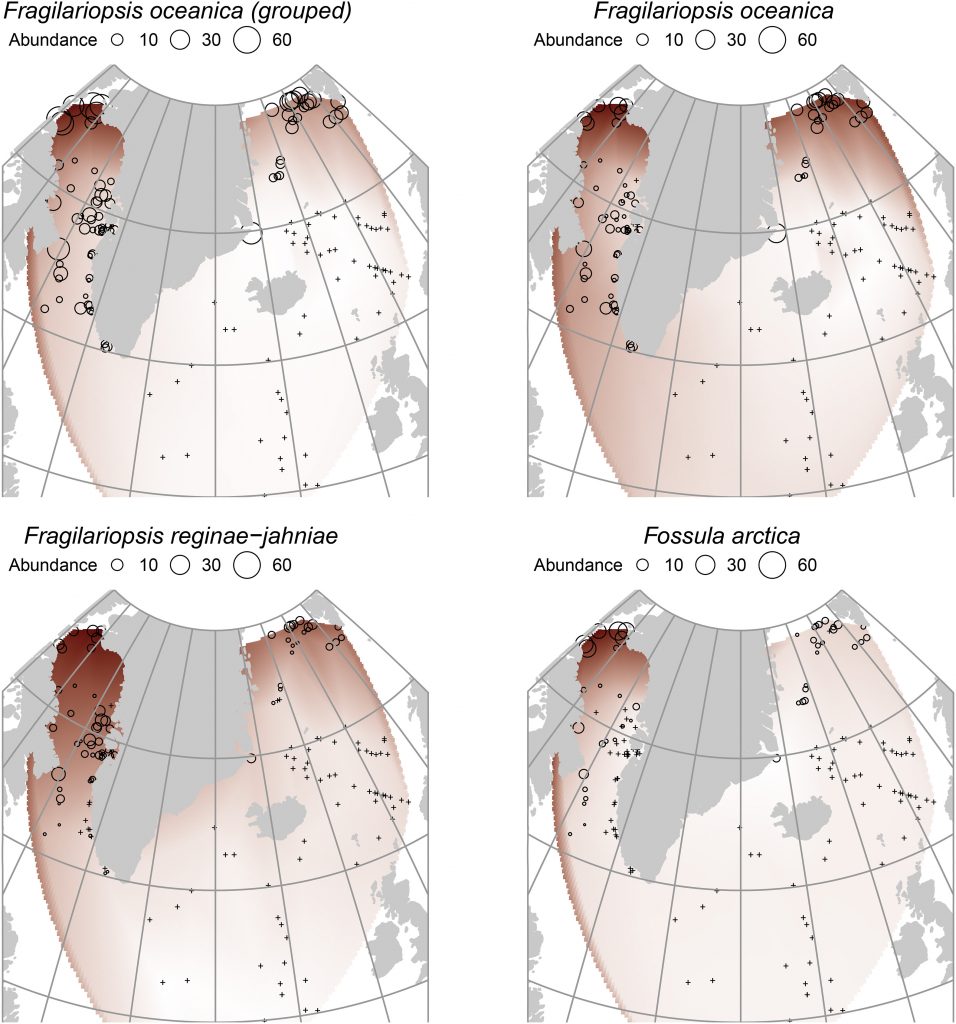
Figure 2. Geographical distributions of the species, grouped and un-grouped. Note the pan-Arctic concentration of F. oceanica compared to F. reginae-jahniae and particularly F. arctica, which is strongly related to the Northwater Polynya region.
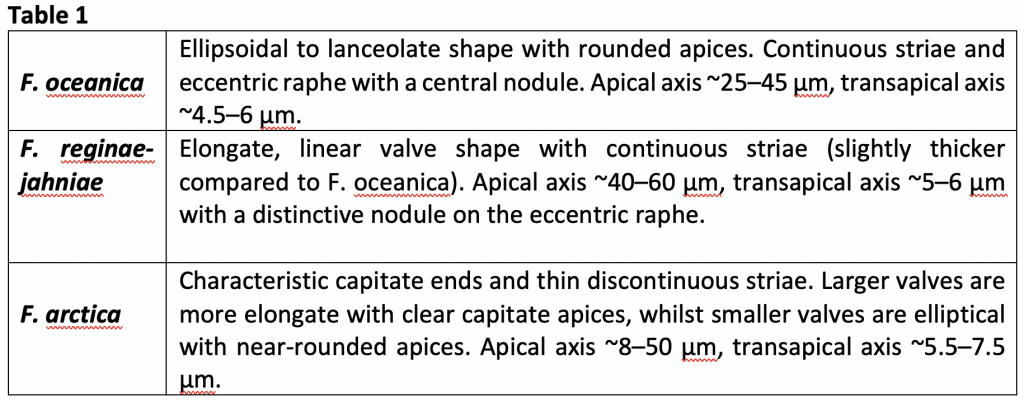
It was apparent from the heterogeneous distributions of the species (Fig. 2) that the individual maximum abundances occurred at different aSST/aSIC. The different respective maxima are described in detail in Table 2, but the general results were: F. oceanica is not a true sea-ice species, being present in a wide range of conditions; F. reginae-jahniae has a strong relationship to high aSIC and cold conditions; F. arctica has a particularly strong relationship to high aSIC and is possibly a specialised species in polynya conditions (year-round open ocean surrounded by sea ice). The differences between the species are significant, particularly of F. reginae-jahniae and F. arctica to F. oceanica. Therefore, separating these species during reconstructions will result in much more nuanced interpretations of palaeo-conditions, particularly of sea ice.
With sea ice declining in the Arctic (9) and the Atlantic Meridional Overturning Circulation weakening (10) , it is imperative that high-resolution reconstructions of past rapid climate change are made from across the Arctic region to better understand what the potential implications may be. Furthermore, sea-ice affiliated algae are a crucial component of the early spring bloom, contributing for 50-60% of total primary production during the transitional phase (11, 12), with pennate diatoms often accounting for >90% of the total biomass (13); it is imperative that we learn more about these keystone species.
____
Ben is a PhD student at RHUL interested in the climatic and ecological implications of polluted sea ice.
List of references:
- Weckström K, Roche BR, Miettinen A, Krawczyk D, Limoges A, Juggins S, Ribeiro S, Heikkilä M., 2020. Improving the paleoceanographic proxy tool kit – On the biogeography and ecology of the sea ice-associated species Fragilariopsis oceanica, Fragilariopsis reginae-jahniae and Fossula arctica in the northern North Atlantic. Marine Micropaleontology 157:101860. https://doi.org/10.1016/j.marmicro.2020.101860.
- Limoges A, Weckström K, Ribeiro S, Georgiadis E, Hansen KE, Martinez P, Seidenkrantz M-S, Giraudeau J, Crosta X, Massé G, 2020. Learning from the past: Impact of the Arctic Oscillation on sea ice and marine productivity off northwest Greenland over the last 9,000 years. Global Change Biology 26:6767–6786. https://doi.org/10.1111/gcb.15334.
- Hernández-Almeida IKR, Bjørklund P, Diz S, Kruglikova T, Ikenoue A, Matul M, Saavedra-Pellitero N, Swanberg, 2020. Life on the ice-edge: Paleoenvironmental significance of the radiolarian species Amphimelissa setosa in the northern hemisphere, Quaternary Science Reviews 248:106565. https://doi.org/10.1016/j.quascirev.2020.106565.
- Armbrecht LH, 2020. The potential of sedimentary ancient DNA to reconstruct past ocean ecosystems. Oceanography 33:116-123. https://doi.org/10.5670/oceanog.2020.211.
- Luostarinen T, Ribeiro, S, Weckström, K, Sejr, M, Meire, L, Tallberg, P, Heikkilä, M, 2020. An annual cycle of diatom succession in two contrasting Greenlandic fjords: from simple sea-ice indicators to varied seasonal strategists, Marine Micropaleontology 158:101873. https://doi.org/10.1016/j.marmicro.2020.101873.
- Koç N, Jansen E, Haflidason H, 1993. Paleoceanographic reconstructions of surface ocean conditions in the Greenland, Iceland and Norwegian seas through the last 14 ka based on diatoms. Quaternary Science Reviews 12:115–140.
- Andersen C, Koç N, Moros M., 2004. A highly unstable Holocene climate in the sub- polar North Atlantic: evidence from diatoms. Quaternary Science Reviews 23:2155–2166. https://doi.org/10.1016/j.quascirev.2004.08.004.
- Miettinen A, Divine D, Husum K, Koç N, Jennings A, 2015. Exceptional ocean surface conditions on the SE Greenland shelf during the Medieval Climate Anomaly. Paleoceanography 30:1657–1674. https://doi.org/10.1002/2015PA002849.
- Polyakov IV, Pnyushkov AV, Alkire, MB, Ashik IM, Baumann TM, Carmack EC, Goszczko I, Guthrie J, Ivanov VV, Kanzow T, Krishfield R, Kwok R, Sundfjord A, Morison J, Rember R, Yulin A, 2017. Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean. Science 356:285–291. https://doi.org/10.1126/science.aai8204.
- Caesar L, McCarthy, GD, Thornalley DJR, Cahill N, Rahmstorf S, 2021. Current Atlantic Meridional Overturning Circulation weakest in last millennium. Nature Geoscience 14, 118–120. https://doi.org/10.1038/s41561-021-00699-z.
- Gosselin M, Legendre L, Therriault J-C, Demers S, Rochet M, 1986. Physical control of the horizontal patchiness of sea-ice microalgae. Marine Ecology Progress Series 29:289–298. https://doi.org/10.3354/meps029289.
- Fernandez-Mendez, M, Katlein, C, Rabe, B, Nicolaus, M, Peeken, I, Bakker, K, Flores, H, Boetius, A, 2015. Photosynthetic production in the Central Arctic Ocean during the record sea-ice minimum in 2012. Biogeosciences 12:3525–3549. https://doi.org/10.5194/bg-12-3525-2015.
- Rozańska, M, Poulin, M, & Gosselin, M., 2008. Protist entrapment in newly formed sea ice in the coastal Arctic Ocean. Journal of Marine Systems 74: 887–901. https://doi.org/10.1016/j.jmarsys.2007.11.009.