Search for predisposing genes for Familial Colorectal Cancer Type X
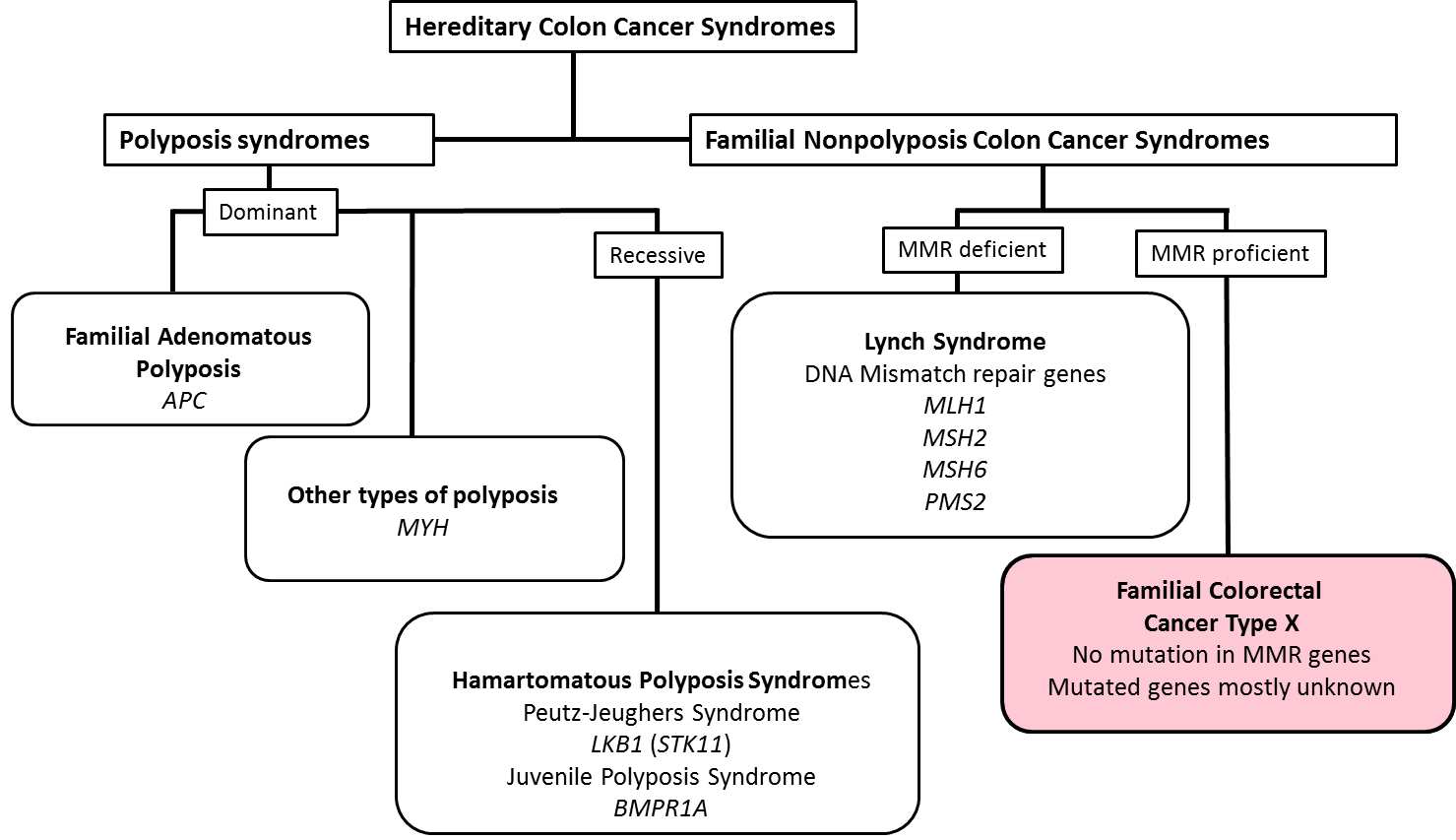
Hereditary nonpolyposis colorectal cancer (HNPCC) as defined by the Amsterdam criteria I or II includes two distinct entities with roughly comparable shares. Families with germline mutations in DNA mismatch repair genes MLH1, MSH2, MSH6, or PMS2 represent Lynch syndrome with over 3,000 predisposing mutations known. Familial Colorectal Cancer Type X (FCCX) is a collective designation for families with no evidence of mismatch repair deficiency, wherein “type X” refers to the as yet unknown nature of predisposition. This project aims to dissect the predisposing defects for the latter families.
Exome sequencing of blood DNA, preferably from multiple affected family members, is our main approach. Exome data are integrated with genetic linkage data as well as tumor data (loss of heterozygosity, aberrant expression, and aberrant DNA methylation) when available. For variants qualifying for predisposing mutations, the mechanisms of pathogenicity will be functionally evaluated.
We discovered novel germline mutations in the gene for bone morphogenetic protein receptor type IA (BMPR1A) in two Amsterdam-positive families from Finland, making 11% of all families investigated (Nieminen et al., 2011, PMID: 21640116). Other BMPR1A mutations have previously been linked to predisposition to the juvenile polyposis syndrome, and our results thus extend the phenotypic spectrum of BMPR1A mutations.
Investigation of a four-generation FCCX family from Finland resulted in the identification of a truncating germline mutation in RPS20, a novel colon cancer predisposition gene encoding a component (S20) of the small ribosomal subunit (Nieminen et al., 2014, PMID:24941021). This is the first report linking germline mutation of RPS20 to hereditary colon cancer susceptibility and predisposition to human disease overall.
Important questions that remain under investigation include:
I. FCCX families with predisposing genes identified
- Genotype-phenotype relationship
- Tumorigenic mechanisms
- Worldwide prevalence of mutations in the genes identified
II. FCCX families with unknown predisposing genes
- Unraveling the predisposing genes and mechanisms by the combined approach described above.
If you are interested in collaboration with us, please contact Päivi Peltomäki at Paivi.Peltomaki@Helsinki.Fi.