An achilles heel in the picornavirus capsid
Our new article on a druggable pocket on the surface of coxsackievirus B3, a major contributor in heart disease is now out in Plos Biology, an open access journal. The study has been picked up by several news outlets including interviews for ScienceNews Science News – Achilles heel in the common cold?, New Scientist, Nordic Life Science, Apteekkari
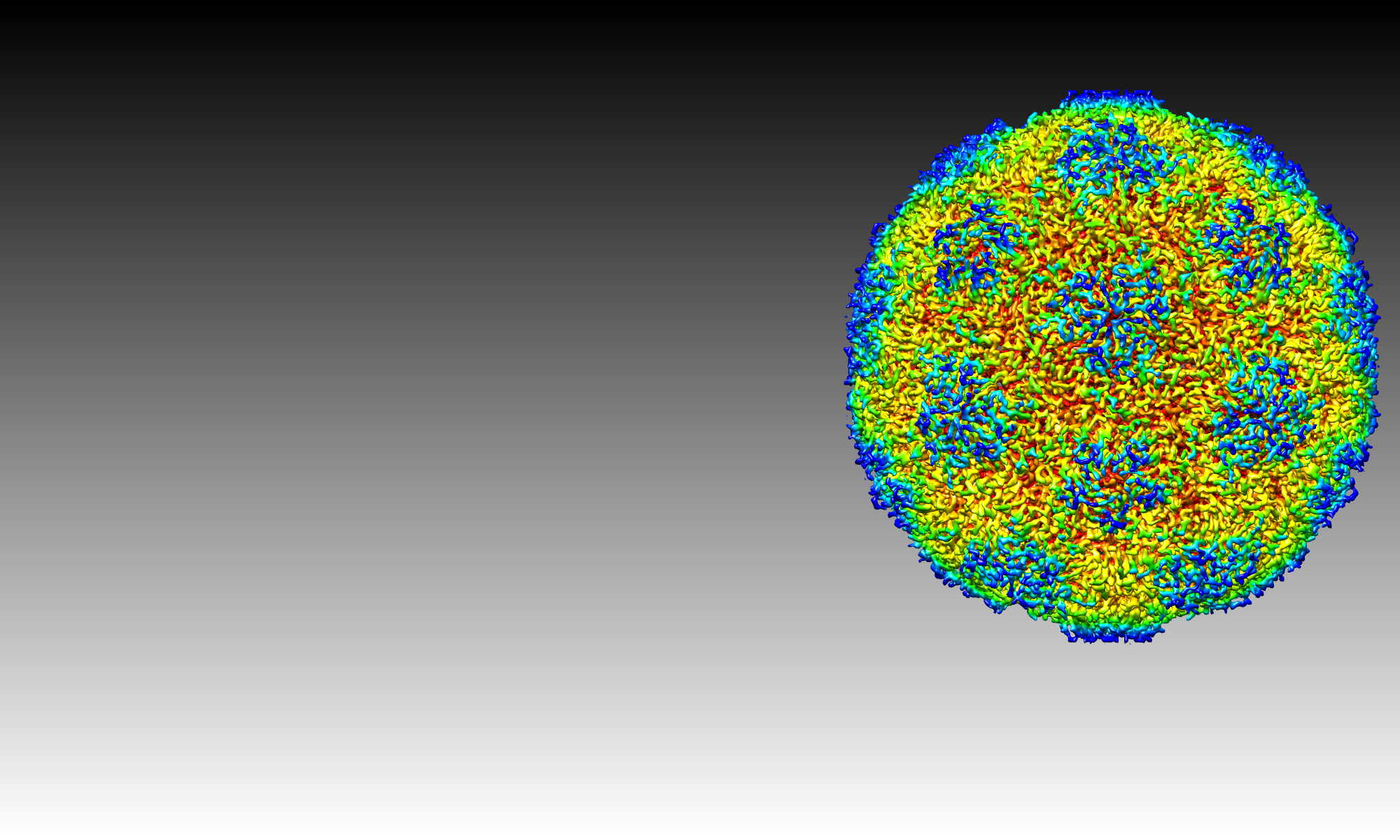
IN THE POCKET A chemical compound (illustrated at center) binds to a pocket on the protein shell (blue, white and magenta) of enterovirus B3, preventing replication. Figure by James Geraets shared This is an open access figure distributed under the terms of a Creative Commons License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.. Please cite
A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses. *Rana Abdelnabi , * James A. Geraets , Yipeng Ma , Carmen Mirabelli , Justin W. Flatt, Aušra Domanska, Leen Delang, Dirk Jochmans, Timiri Ajay Kumar, Venkatesan Jayaprakash, Barij Nayan Sinha, Pieter Leyssen, correspondence to *Sarah J. Butcher , *Johan Neyts
- Published: June 11, 2019
- https://doi.org/10.1371/journal.pbio.3000281

Sarah Butcher awarded Alfred Kordelin prize
The Alfred Kordelin Foundation awarded Sarah Butcher the Alfred Kordelin Foundation prize for her contribution to Finnish science on the 6th November 2017. Link to the announcement in Helsingin Sanomat https://www.hs.fi/kulttuuri/art-2000005437566.html.
Link to the Finnish news in the University web pages https://www.helsinki.fi/fi/uutiset/elamantieteet/alfred-kordelinin-palkinto-professori-sarah-butcherille
Scientists close in on cracking ‘Enigma Code’ of common cold
Our latest work (Shakeel et al Nature Communications 2017; http://rdcu.be/pybT) as reported on www.sciencedaily.com:
************************************************************
The research findings, published in Nature Communications, revealed the workings of a ‘hidden code’ within the genome of Human Parechovirus, a member of the Picornavirus family that includes the common cold, polio, and hand foot and mouth disease.
The work builds on a discovery made in 2015, when scientists at the Universities of Leeds and York, identified a set of encrypted signals in a plant virus with a single-stranded ribonucleic acid (RNA) genome, not too dissimilar to the structure of the Parechovirus that infect humans and can cause sepsis-like illness and meningitis in children.
They found that the details of the decoding mechanism appear identical in all strains of the virus, potentially allowing a single drug to treat them all, something that is not possible with a vaccine.
The team is now working to screen for potential anti-viral drugs that target this decoding mechanism. Successful future partnerships with the pharma-industry and further funding support could potentially see drug development results within the next ten years.
Professor Reidun Twarock, a mathematical biologist at the University of York’s Departments of Mathematics, Biology, and the York Centre for Complex Systems Analysis, said: “Previously scientists have assumed that the signals regulating the assembly of a virus were located in a unique area of the genome.
“Using a combination of biological insight and mathematical modelling, our study suggests that, by contrast, the mechanism relies on multiple dispersed sites in the genome that act together in a cooperative way to enable efficient virus formation.
“The common cold infects more than two billion people annually, making it one of the most successful viral pathogens, so we are excited to make this crucial step forward.”
Scientists had previously attempted to detect assembly signals by genetically recoding these viruses, but failed to find any. The latest results solve this mystery; they show that the additional ‘hidden’ code, responsible for virus formation, is robust against such genome changes, and is conserved across different viruses in the same family.
Professor Peter Stockley, from the Astbury Centre for Structural Molecular Biology at the University of Leeds, said: “The coding works like the cogwheels in a Swiss watch. We now need a drug that has the same effect as pouring sand into the watch; every part of the viral mechanism could be disabled.
“We need to move away from a vaccine approach, which is what we have for flu and polio. Vaccines, although our best source of defence against polio at the moment, can result in the release of more virulent strains of the disease. Protecting against infection therefore relies on continued worldwide vaccination, which is both very expensive and logistically difficult.”
The World Health Organisation has a goal of eliminating polio infections worldwide via vaccination but recognises that before vaccination can be terminated there is a need to develop anti-polio drugs to cure residual infections.
Professor Sarah Butcher, from the University of Helsinki, said: “This new research means that treatment would be less likely to trigger drug resistance, which is currently one of the major problems in anti-viral therapy. This discovery could be a great leap forward in curing a host of conditions.”
***********************************************
Source:
University of York. “Scientists close in on cracking ‘Enigma Code’ of common cold.” ScienceDaily. ScienceDaily, 23 February 2017. <www.sciencedaily.com/releases/2017/02/170223092335.htm>.
Link to paper: http://www.nature.com/articles/s41467-016-0011-z; http://rdcu.be/pybT
Reported elsewhere:
http://us.cnn.com/2017/02/23/health/how-to-cure-common-cold/index.html
http://www.abc15.com/news/national/could-the-common-cold-be-cured
One step forward in developing drugs against sepsis-causing virus in newborns
Resolving the high-resolution structure of a parechovirus by Prof Butcher’s group at the University of Helsinki helps the development of antiviral drugs.
Human parechovirus type 3 is a picornavirus that can cause severe infections in humans, resulting in sepsis and central nervous system disease in newborns. So far the most promising anti-picornaviral drug candidates do not have any effect on the parechovirus, therefore new effective means have to be found.
Researchers in the University of Helsinki have determined a high-resolution structure of the human parechovirus type 3. The three-dimensional model was created by collecting thousands of images of virus with an electron microscope under -190 °C. The images were then computationally aligned and combined.
“The virus genome is a single-stranded RNA, which is encapsidated in a protein shell. About a quarter of the genome is in close contact with the capsid proteins, leading to highly ordered RNA. This has not been seen in other picornaviruses,” describes Postdoctoral Researcher, Dr Shabih Shakeel in the Institute of Biotechnology.
The atomic model of the virus shows a distinct way of how viral proteins interact with each other to stabilize the capsid. The best studied anti-picornaviral drug pleconaril and its derivatives work well against enteroviruses, large group of picornaviruses. The parechovirus type 3 structure demonstrates that pleconaril binding place is blocked in parechoviruses and therefore does not work against this virus group.
Marie Curie Postdoctoral Research Fellow, Dr Ausra Domanska worked on the structure of the same virus in complex with antibody fragments recognising parechovirus type 3.
“In the absence of antiviral drugs, developing broadly neutralising monoclonal antibodies as therapeutic antibodies against this virus is one of the most promising treatment options for clinicians in the near future,” she says.
Human parechovirus type 3 with bound antibody fragments. Picture by Ausra Domanska
_________
Shakeel, S. et al. Multiple capsid-stabilizing interactions revealed in a high-resolution structure of an emerging picornavirus causing neonatal sepsis. Nat. Commun. 7:11387 doi: 10.1038/ncomms11387 (2016) http://www.nature.com/articles/ncomms11387
This study was supported by Academy of Finland, Sigrid Juselius Foundation, People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/ under REA grant agreements no. 612308 and no. 628150. The authors of the study, led by Prof. Sarah Butcher at the Institute of Biotechnology, University of Helsinki, and Dr Wolthers at Academic Medical Center in Amsterdam, are all members of the AIROPico consortium.
AIROPico (Academic Industry R&D Opportunities for Picornaviruses) is the first EU-funded scientific consortium for emerging picornavirus research. Coordinated by Dr Katja Wolthers from the Netherlands, AIROPico brings together researchers from two companies and four academic sites across Europe, and aims to shed new light on how picornaviruses cause disease in humans. The consortium’s objectives include the creation of fast point-of-care diagnostic tools and the development of effective anti-picornaviral treatment.
For more info about the AIROPico project see http://www.airopico.eu/
Sarah Butcher takes up a Professorship in Microbiology from 1st Jan 2016
The Faculty of Biological and Environmental Sciences have hired Sarah Butcher as Professor of Microbiology, and she continues her affiliation with the Institute of Biotechnology (as head of the Structural Biology and Biophysics Programme). On 22nd January she adjudicated over the PhD viva of Teija Ojala (left) with her opponent, Samuel Myllykangas, Blueprint Genetics (middle). The public examination went successfully, pleasing Teija’s two supervisors, Petri Auvinen and Liisa Holm.
Hope for antivirals against parechoviruses
The Butcher, Wolthers and Beaumont labs have recently published an article providing an atomic model of human parechovirus 1. The atomic model was then used to define the epitopes and modes of neutralization for two potential therapeutic antibodies against human parechovirus 1.
Parechoviruses are medically-relevant human pathogens causing mild to severe infections. Currently there are no vaccines or antivirals against them. The Butcher and Wolthers labs as part of the AIROPico consortium in collaboration with the company AIMM Therapeutics, have recently published an article in the Journal of Virology providing an atomic model of human parechovirus 1.
The atomic model was then used to define the epitopes and modes of neutralization for two potential therapeutic antibodies against human parechovirus 1. These human monoclonal antibodies show cross-neutralization against other human parechoviruses as well.
The AM18 antibody recognizes arginine-glycine-aspartic acid motif present on viral capsid protein 1 and neutralizes the virus by competing with the cellular receptor which also recognizes the same motif. The AM28 antibody neutralizes by binding to a conformational epitope and blocking the viral RNA release site.
“This is a first step towards developing therapies against parechoviruses which is one of the long term goals of AIROPico,” says Research Director Sarah Butcher.
The study was supported by the Seventh Framework Programme of the European Union AIPP under contract PIAPP-GA-2013-612308 (AIROPico), the Academy of Finland, the Sigrid Juselius Foundation, the Netherlands Organisation for Health Research and Development, The AMC Research Council, the European Molecular Biology Organization and the European Society of Clinical Virology.

Fab molecules bound to human parechovirus 1.
Picture copyright owned by Pasi Laurinmäki.
A new spin on measles virus matrix
Measles is an important disease worldwide that is highly infectious, causing the deaths of over 100000 people annually. We used advanced electron microscopy and image processing methods to make a a three-dimensional model of measles virus. The new model helps to explain many previous, unaccounted for observations in the life cycle of the virus. Measles virus belongs to a family of viruses whose members are all pleomorphic enveloped viruses. All the members of this family contain a so called matrix protein which in measles virus coats the ribonucleoparticle making a “rigatoni-like” helix. More details from the original article by Liljeroos et al in the Proceedings of the National Academy of Science (USA)
LDL particles and heart disease
Low-density lipoprotein (LDL) particles are the major carriers of cholesterol in the human circulation. They have key roles in both cholesterol physiology and in the development of atherosclerosis. Together with researchers from the Wihuri Research Institute, Aalto University and Oulu University we characterised the structure of LDL particles at the human body temperature to understand the organisation of the lipids and protein that they are made from.See the article for more details
Kumar, V., Butcher, S.J., Öörni, K., Engelhardt, P., Heikkonen, J., Kaski, K., Ala-Korpela, M., Kovanen, P.T. (2011) Three-dimensional cryoEM reconstruction of native LDL particles to 16å resolution at physiological body temperature. PLoS ONE 6(5): e18841. doi:10.1371/journal.pone.0018841.
Development of photoactivated drugs
Efficient drug delivery to the intended target is one of the biggest challenges facing the pharmaceutical industry today. Together with researchers from the Faculty of Pharmacy, we are trying to develop drugs that can be activated by pulses of light in different tissues. See more in the article by
Paasonen, L. Sipilä, T., Subrizi, A., Laurinmäki, P., Butcher, S.J., Rappolt, M., Yaghmur, A., Urtti, A., Yliperttula, M. (2010) Gold-embedded photosensitive liposomes for drug delivery: triggering mechanism and intracellular release. J. Controlled Release 147:136-143