The Annual Meeting of the Finnish collaborative HNPCC reseach groups was organized in Jyväskylä on 9-10th of March.
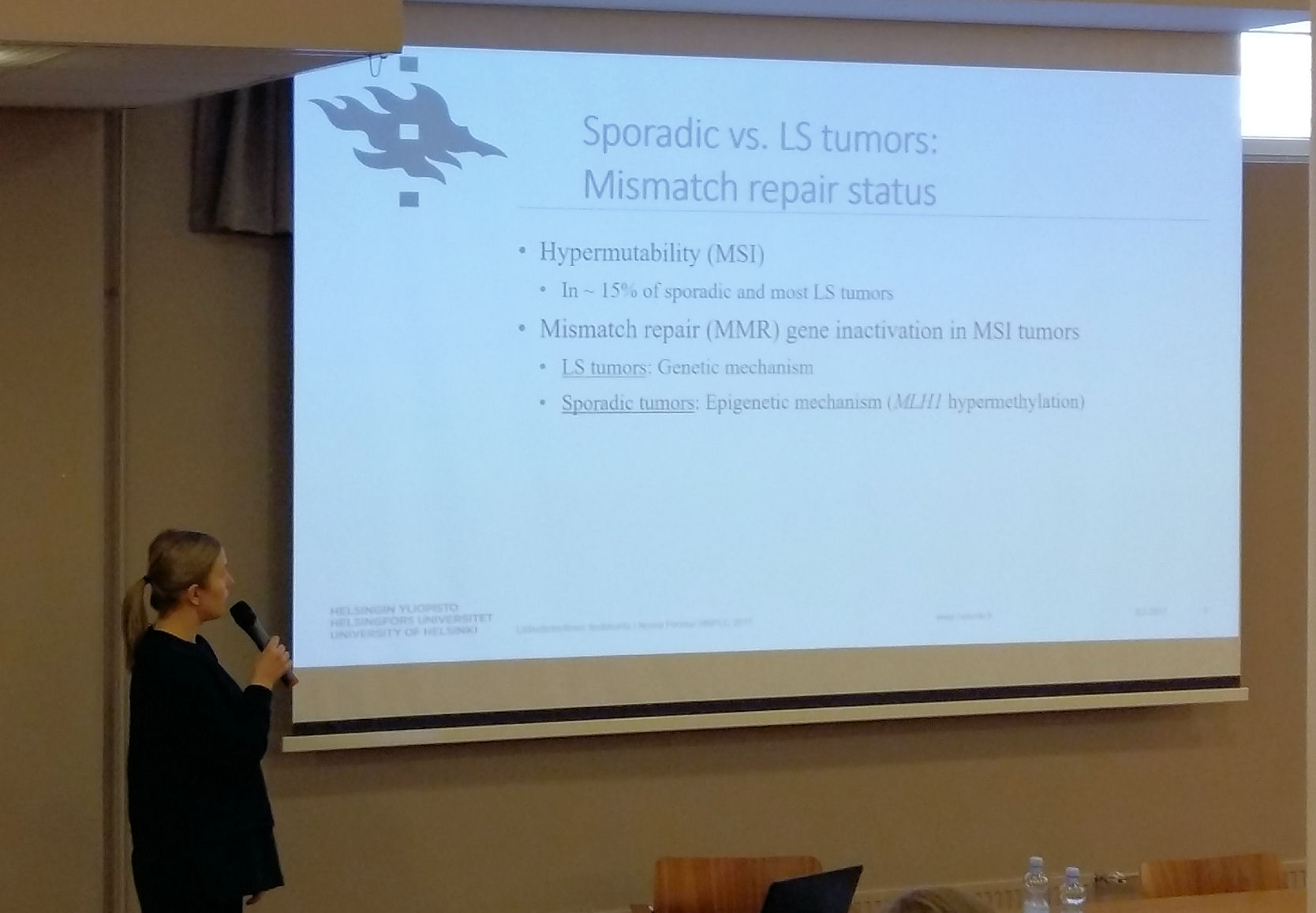
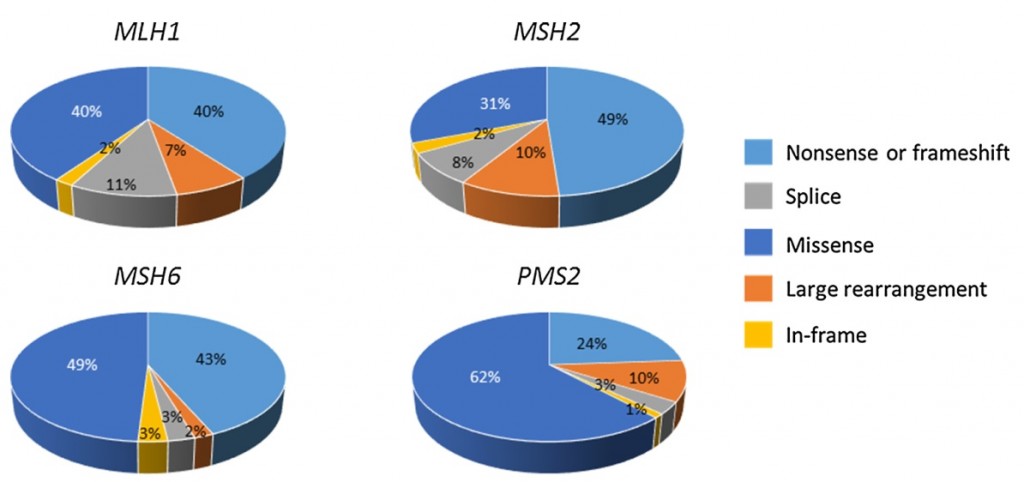
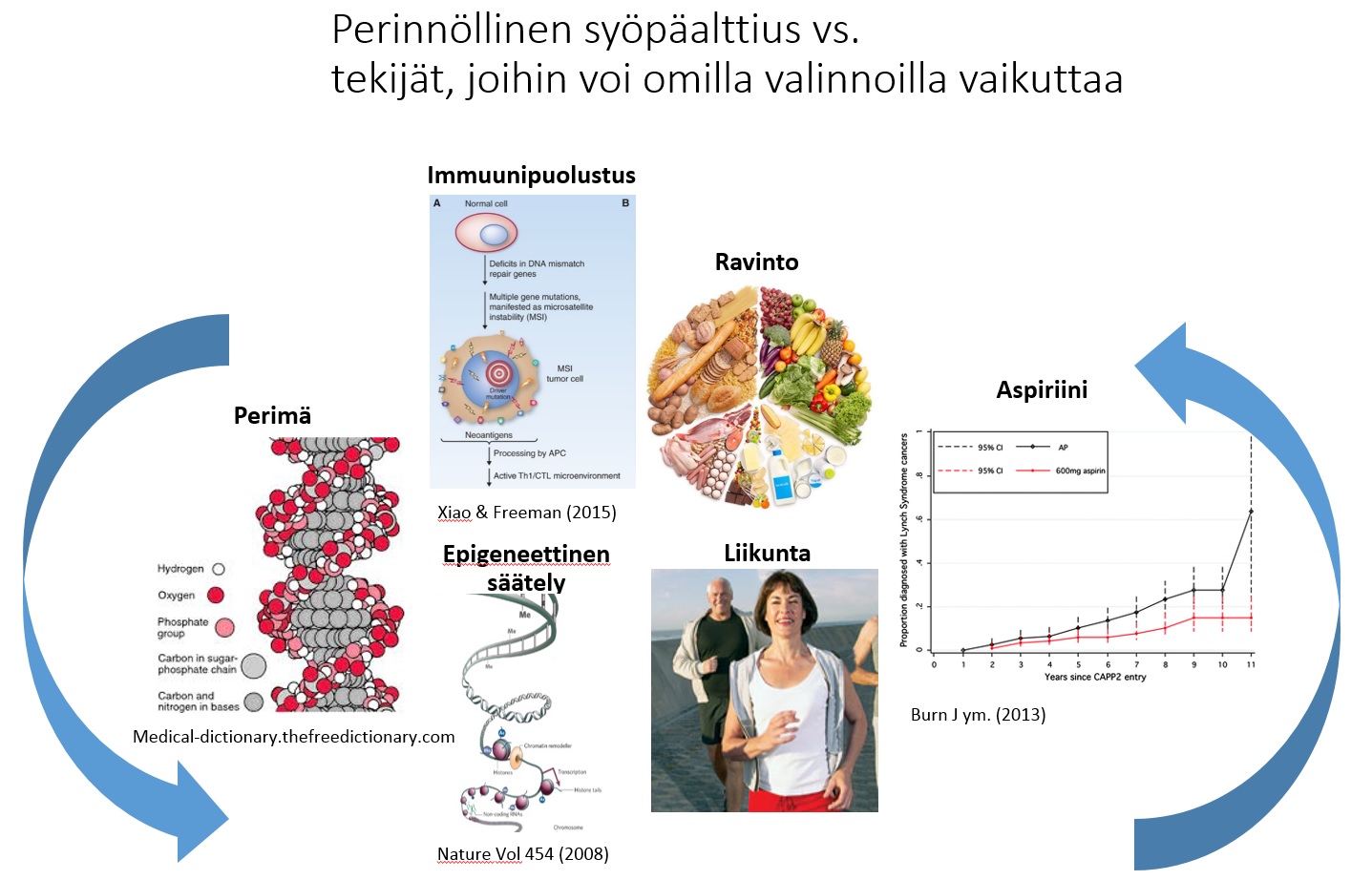
Prof. Päivi Pelomäki gave an overview of the on-going research projects including the collaborative project initiated together with Prof. Jukka-Pekka Mecklin and Prof. Minna Nyström funded by Jane and Aatos Erkko Foundation in aim to recognize molecular mechanisms of colon cancer susceptibility. Satu Valo, PhD, and Noora Porkka, M.Sc, presented projects related to epigenetic and genetic characterization of Lynch syndrome related cancer. Minttu Kansikas, PhD, and Mariann Kasela, M.Sc, from Prof. Minna Nyström’s research group presented on-going projects related to the development and validation of a non-invasive test to detect Lynch syndrome (http://www.lscancerdiag.com/) and assessment of the effects of PMS2 gene function on MMR repair efficiency.