For the past several decades, forestry has been interested in developing transgenics to improve wood production. This is because in the majority of vascular plants, lignin biomass averages 20-30%, which means there is a large energetic and monetary cost to remove this unwanted lignin, a hydrophobic molecule with strong covalent bonds, during processing (Robinson, 1990). But despite its cost to paper and pulp mills, lignin has exciting potential applications as an organic molecule in the pharmaceutical, construction, and packaging fields, among others (Albuquerque et al., 2021). With proper bioengineering, lignin could even be used as a biomaterial capable of replacing fossil fuels in plastic production. However, current research and knowledge of lignification, the process wherein lignin is deposited in the plant, is lacking when it comes to our ability to produce widely-commercially viable plants with manipulated lignin properties. This is where the small, bendy shrub, Eastern leatherwood, enters the picture.

During the course of my four months with the Fagerstedt lab, I had the opportunity to work with leatherwood, a species that was recently discovered to have unique lignin patterning, in a completely novel way (Mottiar et al., 2020). While I knew that I would likely use antibody staining to identify the locations of molecules, such as pectin, I didn’t know that I would have the opportunity to use a transmission electron microscope, or to turn this research into a thesis project.
One of the best things about science, and this traineeship with HiLIFE, is that I was able to try things that I, nor anyone else, had ever done before. With Yaseen’s expertise, the post-doc supervising me, I went from researching how to use a transmission electron microscope, which sends a particle beam through your ultra thin sample, allowing you to see where electrons are able to pass through, and where they are blocked, to actually embedding samples in small, pill-shaped resin capsules and eventually imaging those samples on a machine that looks like a spaceship (the transmission electron microscope). This type of microscopy allowed us to see, in extremely fine detail, the cell wall of leatherwood tissue. While the image below has some sample flaws (the dark line is a wrinkle in the wood section, and a few of the cell walls have torn), I think it’s a really cool way to see the cambium (with inner cellular contents that have been destroyed) and the empty xylem cells that, in a living tree, would transport water. Below is a sample of leatherwood, and if you look at the area between the cell walls, or middle lamella, you can see that it’s lighter in color, indicating that there is not staining present, and therefore there is not enough lignin present to darken the area.
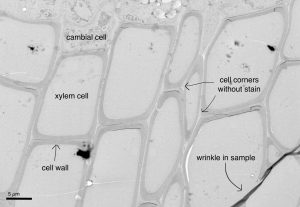
I’m so grateful for the opportunity to spend four months of full-time work trying out different wet lab techniques and learning about what it is like to be a full time researcher. Additionally, this research has provided a step further in the quest to generate transgenic trees with modified lignin content and distribution. Hopefully in the future, scientists will be able to modify commercial species with lignin in ways that allow lignin to be used to replace plastics, among other things.
Works cited
