Laitinen seeks to understand hunger-satiety signalling to understand its root causes.
Check the introduction video where she describes her project.
Suvi Laitinen, 21, researcher and MD-PhD-student from the University of Helsinki started meticulously chasing her hypothesis about elevated hunger/decreased satiety in the human brain in 2022. She co-founded the Obesity Curiosity research project with Dr Teemu Aitta-Aho (PI of the Loss of Control Behaviour lab, Department of Pharmacology, University of Helsinki). She explains the big idea behind Obesity Curiosity:
“It seems that this effect [of environmental factor(s)] causes us to eat a tiny bit more every day. I hypothesise people have decreased satiety, increased hunger, or both. Some people seem to be more vulnerable to the effect while others are more resilient. This would explain why weight management is gruelling. The urge to eat is one of the most powerful signals of the body and almost impossible to resist.”
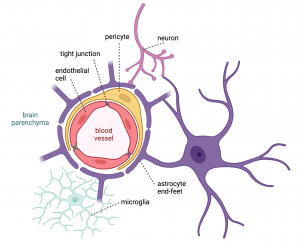
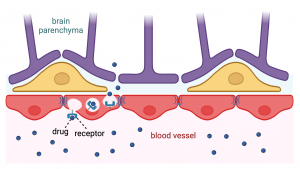
Laitinen’s PhD project is part of Obesity Curiosity and her goal in the first stage, is to develop a human arcuate nucleus model. Then in the second stage, they will use the model to study the effect of factors of interest, such as medications with weight gain as a side effect, on the human brain in vitro.
Currently, Laitinen is completing her research at a scientific collaboration visit in Dr Florian Merkle’s lab at the Institute of Metabolic Science University of Cambridge, funded by the Helsinki Institute of Life Science (HiLIFE) Undergraduate Student Research Trainee Scholarship. “My work here is focused on directed differentiation of human induced pluripotent stem cells (hiPSC) to hypothalamic neural origin cells, including neurons. This way we can generate a model that quite faithfully replicates human physiology without having to deal with the inaccessibility of the human brain otherwise. The hiPSC cells have been generated from volunteer donations of patients in past”, she explains.
“Working in Dr Merkle’s lab allows me to learn their robust methodology of hiPSC-derived 2D hypothalamic cell culture and with his generous support I am highly grateful of, I have expanded into preliminary testing of my own protocol to generate organoids. I hope to be able to establish corresponding cultures when I return to Helsinki. The research methods learnt during the exchange will be the firm foundation of my PhD research project, and forming strong collaboration will be immensely helpful. I am highly enthusiastic about this opportunity!”
Laitinen independently designed her new research opening involving a novel methodology of assembloids based on the literature of the field in 2022 and convinced Dr Aitta-Aho to become her supervising Principal Investigator in October of the same year. She has since executed her research in the Loss of Control Behaviour lab of Dr Aitta-Aho alongside her medical school studies at the University of Helsinki. Obesity Curiosity, the wider project involving multiple approaches to study the root causes of the obesity pandemic is their joint effort.
“To understand obesity, I have started the same way as many scientists before me, by finding special cases and trying to understand the general rule from there. Common clinical knowledge and scientifically proven fact is that many psychiatric drugs statistically cause patients to gain substantial amounts of weight in a relatively short period of time. I want to study this with a novel human brain model. Long-term, I hope to be able to help in improving the treatment and prevention of obesity”
Before founding Obesity Curiosity, Laitinen had close to nine months of full-time biomedical research experience. After winning Millenium Youth Prize 2018 , she worked at Aalto University in the Department of Neuroscience and biomedical engineering rotating between different laboratories in summer 2019, exploring what the work of a researcher is like. Summer of 2020 and the four months between final exams and starting medical school in 2021, she worked at Professor Pekka Katajisto’s lab (HiLIFE Institute, University of Helsinki) in stem cell research.
In Katajisto lab, Laitinen investigated basal mammary gland cell adhesions to EMC proteins in the normal development and the cancer of mammary gland epithelium.
“The experience was amazing! Professor Pekka Katajisto and Dr Johanna Englund, my supervising researcher in the lab, always believed the most and best of me, teaching me to reach my full potential and ask all my questions. They also demonstrated how great supervising at best can be!”
Her work in 2021 also resulted in her first two co-authored publications, an accomplishment she is proud of and grateful for. “In the scientific world, they are proof that I am capable of executing research at the meticulous level, and maybe help to get respected as a fellow researcher regardless of my young age”
In her first year of medical school, she got accepted to the MD-PhD-program at the University of Helsinki, which had been her goal ever since she learnt that it is possible to become physician-doctor. “It was a nerve-wracking process, as one can apply for the program only once [in the first year of medical school] and I had focused my work towards that goal for a long time. Acceptance felt like reaching the top of a mountain – a completion – and a wonderful landscape of the research world opening in front of me!”
Her initial interest in cancer led to her choosing the first rotation lab studying ovarian cancer. She completed 2 months rotation in the Dr Anniina Färkkilä’s lab (Systems Oncology Research Program (ONCOSYS), University of Helsinki) in the summer of 2022 to learn about three cutting-edge methodologies: cyclic immunofluorescence method, patient-donated sample-derived organoids and complex cell typing flow cytometry.“The point of rotation is to get experience that helps with PhD projects, and I strongly believe these methods will help me design my own project and readouts to investigate my research questions”
“The burning question keeping me up at night is What causes the obesity pandemic? Ultimately this is about the health of over 700 million people suffering from obesity already today and countless others to come. Obesity affects everyone, if not directly themselves, their loved ones. I have a hypothesis, so I am trying my best to test it. I am working in molecular neuroscience to hopefully make the world a better place.”
Laitinen was awarded HiLIFE Undergraduate Student Research Trainee Scholarship for international research exchange in November 2022. She secured a position in Dr Merkle’s lab to conduct the research visit between May-August 2023 working on her Obesity Curiosity project.