Imagine an agency headquarters with tight security policies in place. There are security guards on all doors and no entry without an ID badge. Various employees pass by the gate, all with different job titles and responsibilities. Entrance is also granted for the supportive personnel such as maintenance, cleaning services, and catering. Everybody knows their own specialized role and together with a strong management it is made sure that everything flows in a highly organized manner. Occasionally, an intruder tries to invade the establishment, but they are swiftly stopped by the security and police officers who arrive just a moment later to make sure nothing was stolen, and no one harmed. And if something or someone was to be injured, a team of healthcare professionals would arrive to take care of the situation.
In a similar way, in our bodies, molecules are travelling in the bloodstream and when wishing to enter the brain they arrive to the gate of the blood-brain barrier, or shorter the BBB. During my HiLIFE Research Trainee internship, I got an opportunity to join the Brain Targeting Program at the Wyss Institute for Biologically Inspired Engineering at Harvard University. Research in this translational program centers around the study of the BBB.
What is the Blood-Brain Barrier?
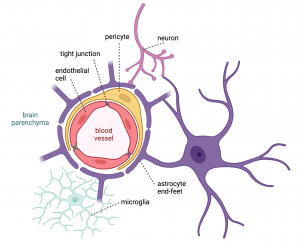
The BBB is a highly specialized structure consisting of endothelial cells that form the blood vessel wall or lumen. These cells are located very close to one another as they form the so-called tight and adherens junctions by binding to multiple proteins. This compact structure allows a limited number of molecules to pass through the BBB. Molecules can pass through via diffusion only if they are small enough or lipid soluble. Another mechanism to cross the BBB is by utilizing transport molecules which carry some substances from the blood to the brain parenchyma (that is, the functional tissue of the brain). Thus, only if the molecule has a correct ID badge, it can enter to the brain.
In the BBB, on the brain side, endothelial cells are enclosed by other cell types: pericytes and the end-feet of astrocytes that support the integrity of the barrier – similar to those security guards in the agency headquarters. To protect the brain from the occasional invasion of intruders, there are microglial cells which work with astrocytes to engulf unwanted molecules or injured cells, and produce cytokines which amplify inflammatory signals to increase the body’s response to the intruder. There is also an efflux mechanism that can expel the uninvited guests who manage to slip through the BBB. Not only do astrocytes contribute to the integrity of the BBB, but they also connect neurons to the vasculature. Thus, there is dynamic communication between the blood vessels and the brain to keep the entire structure highly functional – in our agency example they function like a network of guards with their security earpieces.
Together, the BBB with all the different cell types form the neurovascular unit. This specialized structure is distinct from any other blood vessels in the body, and it therefore also functions very uniquely. It maintains the regulation of blood, oxygen and nutrient flow, immune response, and waste clearance which are all crucial for the homeostasis – the self-regulated normal functioning – of the brain and the body.
The Challenge for Delivery of Therapeutics into the Brain
As much as the BBB protects the brain from unwanted guests such as microbes and toxins, it also very effectively inhibits various drug molecules from reaching the brain. The entry of up to 98% of small-molecule drugs and 100% of larger macromolecular therapeutics is blocked by the BBB. This makes it difficult to treat different diseases affecting the central nervous system, such as neurodevelopmental and neurodegenerative diseases or brain tumors.
Various methods for enhancing brain drug delivery have been developed over decades, yet only a few have proven effective. Some of these technologies need surgery or other invasive methods such as deep brain stimulation, while others such as receptor-mediated, nanoparticle carrier, or focused ultrasound strategies are non-invasive. Our Brain Targeting Team at the Wyss Institute focuses on target discovery and validation utilizing the non-invasive receptor-mediated method, named as receptor-mediated transcytosis, for more efficient brain drug delivery. My main tasks involve handling human brain tissue samples and analyzing both transcriptomic and proteomic data to identify and assess the transport potential of numerous targets.
Receptor-mediated transporters are endogenous proteins located at the BBB blood vessel wall that can have various functions in the body. For example, the most studied receptor-mediated transporter, the transferrin receptor, normally functions in iron uptake from the cell membrane inside the cell. Antibodies can be engineered to bind to these receptors in a way that also other molecules than only the endogenous ones can be internalized. Antibodies linked with desirable therapeutics and designed to utilize these transporter targets are often referred to as Trojan horses, as they are only allowed to cross the BBB when in disguise, subsequently exerting their therapeutic effects once inside the brain. Thus, transferrin receptors can recognize an antibody while inadvertently allowing the entire complex to be carried in the brain without the body’s surveillance system recognizing the foreign drug molecule.
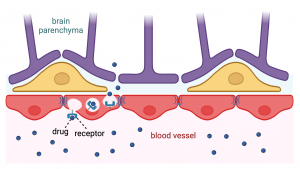
I prefer to imagine that the BBB and therapeutic compounds are on the same side of the battle. Thus, instead of a Trojan horse, this technology harnesses the capabilities of our body so that the BBB offers a helping hand in drug delivery and welcomes an additional aid to battle against protein aggregates found from neurodegenerative diseases or tumor cells in brain cancers when our body and its cells need assistance. Ultimately, it is about granting an ID badge for the drug, for the invited guest in the headquarters of our wondrous body.
A little bit about me:
I am a Master’s student in Translational Medicine at University of Helsinki, currently located in Boston, United States, to conduct a six-month-long internship at the Wyss Institute at Harvard University. In my M.Sc. studies I specialize in translational neuroscience and personalized medicine. My Master’s thesis looked into the molecular mechanisms behind Alzheimer’s disease and while in the midst of my experiments, I got enchanted about the BBB. Therefore, I am deeply thankful and excited to contribute to the Brain Targeting Program at the Wyss where innovations are translated into clinical practice. My next HiLIFE Trainee blog post will provide more insights into my internship experiences, so stay engaged for more!
Mareena Hyypiä


























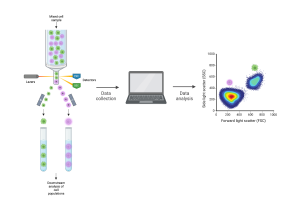 [Flow cytometry schematic. Cells are sent through the flow cytometry instrument one by one. The machine detects their properties and sends them to a computer for analysis. The cells can be separated by type for downstream analysis. Created with
[Flow cytometry schematic. Cells are sent through the flow cytometry instrument one by one. The machine detects their properties and sends them to a computer for analysis. The cells can be separated by type for downstream analysis. Created with