Hello everybody!
My name is Rosa López, and I am delighted to be one of the 2022 HiLIFE Research Trainees. In this post, I would like to introduce who am I and what took me to this life stage, as well as my interest in science and my current research topic. That said, let’s go deep into the matter!
Who am I and how did I arrive at this point?

I am a young scientist who graduated in Biomedical Sciences at the University of Barcelona in 2019. However, my last year of the degree did not take place in my hometown since I was granted an Erasmus Scholarship to carry out my final degree project at the University of Helsinki. For nearly a year, I got to study the molecular mechanism of ageing in Saccharomyces cerevisiae, also known as yeast; in Prof. Juha Saarikangas’ laboratory. One year later, when I was already addicted to Helsinki and its landscapes, I got accepted into the Master’s program of Genetics and Molecular Biosciences (GMB) at the University of Helsinki. Within this program, I decided to pursue the Cell and Developmental Biology study track since my main interests lie in stem cell biology, regenerative medicine, and cellular ageing. Several lectures about regulation mechanisms of stem cells were enough to encourage me to search for laboratories whose main research topic was stem cell regulation. This decision led me to my current point, I am a Master’s thesis student at the Laboratory of Stem Cell Bioengineering (LSCB) at EPFL, the Swiss Federal Institute of Technology in Lausanne. I was thrilled when Prof. Matthias Lütolf awarded me with this position at his laboratory. Additionally, for this traineeship, I was awarded the HiLIFE Research Trainee Scholarship issued by the Helsinki Institute of Life Sciences (HiLIFE) which provides financial support, as well as promotes scientific communication to the public. I would like to state that I am highly grateful to the Selection Committee for considering me as a fit candidate for this position.
What am I exactly doing at the LSCB and what is my main research topic?
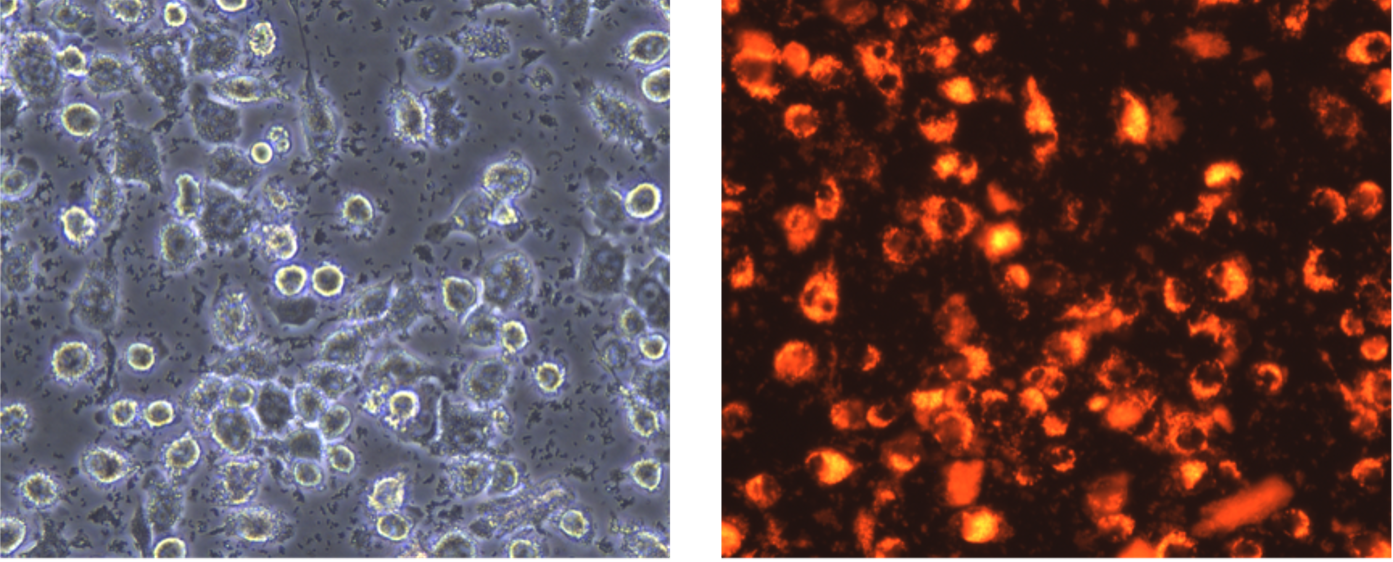
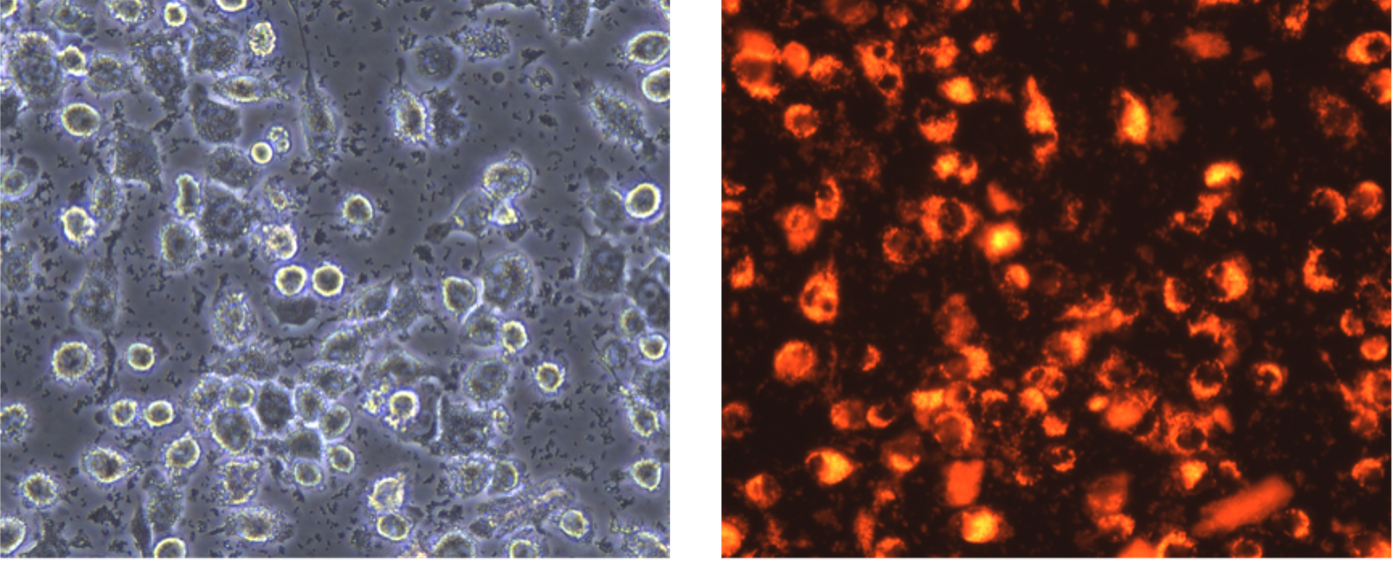
I would like to start this part with a bit of scientific background to give you a grasp of the whole picture. Nowadays, the term ‘organoids’ is quite known in the scientific community and refers to a 3D multicellular in vitro tissue construct that mimics its corresponding tissue in vivo1. Although organoids are being used around the globe and for several purposes, there are some limitations in this methodology. First of all, since they are a 3D structure, they are usually embedded in a Matrigel matrix whose origin is from a mouse sarcoma basement membrane and has a high batch-to-batch variability2. The Matrigel origin abolishes the possibility of its usage for regenerative medicine in humans, and the batch-to-batch variability interferes with the experiment reproducibility. Additionally, while some organoids are more characterized than others due to their tissue of origin, none of them keeps a physiological tissue shape, nor an unlimited functionality or a high lifespan3. To solvent these limitations on organoid culturing, the LSCB laboratory’s main research goal is to develop third-generation organoids from stem cells by using innovative bioengineering strategies.
My contribution to the lab’s main goal is to test the effect of different ECM proteins on gastric stem cell differentiation and regulation. Human gastric organoids are not as well characterized as human intestinal organoids, as a matter of fact, not all the cell components of the human gastric glands are able to be differentiated in the common 3D organoid model4. On the other side, the focus on the extracellular matrix (ECM) as a key niche component of stem cells has exponentially increased in the past years5,6. Therefore, I am researching whether bioengineering a synthetic hydrogel enriched with different ECM proteins can modulate human gastric stem cell regulation and differentiation to improve the pre-existing 3D organoid model.
Even though I started this journey last November, it is still not finished! Impressive things are yet to come, and I expect to have interesting results by the end of this internship. I will keep you posted! In the meantime, you can also check HiLIFE Research Trainees’ social media for more daily life stories 🙂
I am particularly obsessed with citing, so down below you have some references of what I just stated in case someone wants to go deeper on the topic!
- Souza, D. N. (2018, January 3). Organoids. Nature. Retrieved February 23, 2022, from https://www.nature.com/articles/nmeth.4576?error=cookies_not_supported&code=14fb20f7-ab18-46ff-8850-9eaae3e3281e
- Serban, M. A., & Prestwich, G. D. (2008). Modular extracellular matrices: Solutions for the puzzle. Methods, 45(1), 93–98. https://doi.org/10.1016/j.ymeth.2008.01.010
- Hofer, M., & Lutolf, M. P. (2021). Engineering organoids. Nature Reviews Materials, 6(5), 402–420. https://doi.org/10.1038/s41578-021-00279-y
- Seidlitz, T., Koo, B. K., & Stange, D. E. (2020). Gastric organoids—an in vitro model system for the study of gastric development and road to personalized medicine. Cell Death & Differentiation, 28(1), 68–83. https://doi.org/10.1038/s41418-020-00662-2
- Pardo-Saganta, A., Calvo, I. A., Saez, B., & Prosper, F. (2019). Role of the Extracellular Matrix in Stem Cell Maintenance. Current Stem Cell Reports, 5(1), 1–10. https://doi.org/10.1007/s40778-019-0149-9
- Rezakhani, S., Gjorevski, N., & Lutolf, M. (2021). Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials, 276, 121020. https://doi.org/10.1016/j.biomaterials.2021.121020